1. Background
The prevalence of thyroid cancer is increasing worldwide as a result of the increased use of diagnostic imaging (1). Thyroidectomy and dissection of the central compartment of the neck are the initial stages of thyroid cancer treatment in most cases. In cases where thyroid cancer is biologically less aggressive in patients under 45 years of age, thyroid-preserving surgery can be used (2). Over the past decade, the management of advanced thyroid cancer has experienced significant advancements, leading to the approval of some drugs for each subtype of thyroid cancer. Nevertheless, the effectiveness of these drugs in patients remains unsatisfactory due to both primary and secondary drug resistance (3, 4).
Some plants contain anti-cancer agents, whose derivatives have been proven useful for the treatment or prevention of human cancers (5). In the 1950s, scientists began to systematically investigate natural compounds as a source of useful anticancer agents. Recently, it has been suggested that the use of natural products has been the most successful strategy in the discovery of new drugs (6). The anticancer potential of many plants has been investigated in vitro, but there is a long gap between promising in vitro results and their use as medicine (7).
Dioscin is a natural steroid saponin, present in high levels in legumes and Dioscoraceae. It serves as the fundamental raw material for the production of steroid hormone medications and steroid-based contraceptives. In the past few decades, some studies have been conducted on its medicinal properties. Dioscin has shown anticancer, lipid-regulating, anti-platelet aggregation, and bile-enhancing effects. It can be used as a medicine to treat cardiovascular diseases, encephalitis, dermatosis, and tumors (8).
The PI3K/AKT/mTOR pathway serves as a crucial signaling mechanism in cellular functions, including growth and proliferation, motility, survival, and apoptosis. Elevated activity within this signaling pathway contributes to the growth and proliferation of cancer cells across various human cancers (9). The phosphorylated variant of phosphatase and tensin homolog (PTEN) deleted on chromosome ten functions as a tumor suppressor by inhibiting PI3K activity. The PTEN facilitates the dephosphorylation of PIP3, resulting in the activation of the PI3K/AKT pathway. In cancer cell lines, PTEN undergoes phosphorylation and is significantly inhibited by the activation of PI3K/AKT (10). Induction of PTEN inhibits the growth of cancer cells and prolongs the survival of mice with disseminated peritoneal tumors (11).
The Wnt/β-catenin signaling pathway is crucial in the process of tumorigenesis. DACT1 expression is diminished in various tumors, yet it is found to be overexpressed in certain other tumor types. The dysregulation of DACT1 has been linked to unfavorable prognoses in cancer. Furthermore, DACT1 has been identified as a novel inhibitor of the WNT/β-catenin signaling pathway in hepatocellular carcinoma (12). However, its exact biological functions in cancer pathogenesis are unknown.
2. Objectives
Considering the key role of PTEN and DACT1 in the apoptosis process and the importance of targeting these genes in cancer treatment, the current research sought to examine the influence of dioscin on the expression levels of PTEN and DACT1 genes, as well as its impact on apoptosis in thyroid cancer cells.
3. Methods
3.1. Cell Culture
This research was an in vitro study carried out in the cell culture laboratory of the Department of Anatomy, School of Medicine, Kermanshah University of Medical Sciences. The B-CPAP thyroid cancer cell line (Pasteur Institute, Tehran, Iran) was cultured in T75 flasks containing DMEM/F12 supplemented with 10% FBS without antibiotics. The cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2 (13). A stock solution of dioscin (Sigma-Aldrich, Missouri, USA) at a concentration of 10 mg/mL was prepared using dimethyl sulfoxide (DMSO) (Sigma-Aldrich, Missouri, USA). The final concentration of DMSO did not exceed 0.2% (v/v) in the culture medium.
3.2. Viability and Cytotoxicity Tests
The cells were plated in 96-well plates (15 × 103 cells/well), incubated overnight, and subsequently treated with different concentrations (1.25, 2.5, 5, 10, 20, 40, and 80 μM) of dioscin for durations of 24, 48, 72, and 96 hours. Subsequently, the MTT assay was conducted following the methodology outlined in a prior research study (14), and the half-maximal inhibitory concentration (IC50) values were determined using GraphPad Prism (GraphPad Software, San Diego, California) version 9.1.0.
For toxicity measurement, a lactate dehydrogenase activity assay was used. After treatment, the activity of the lactate dehydrogenase enzyme was measured using the colorimetric method and a specific kit (Pars Azmon, Tehran, Iran) according to the kit protocol.
3.3. Apoptosis Assay
After treating the cells with the IC50 concentration of dioscin for 24 hours, they were collected, centrifuged, and washed with sterile PBS buffer. Then, 100 µL of Binding buffer (Sigma-Aldrich, Missouri, USA) was added to the sediment of the cells in 1.5 mL microtubes. Subsequently, 10 μL of propidium iodide (PI) (Sigma-Aldrich, Missouri, USA) dye and 5 μL of annexin-V (Sigma-Aldrich, Missouri, USA) dye were added to the microtubes. All the contents were mixed slowly. The samples were allowed to incubate at room temperature for 10 minutes in a dark environment. Finally, cell analysis was conducted by flow cytometry.
3.4. Gene Expression Analysis
After treatment with the IC50 concentration of dioscin for 24 hours, the expression levels of DACT1 and PTEN were analyzed by real-time PCR following the methodology outlined in a prior research study (15). All the primer sequences were designed using GeneRunner software (Hastings Software, Hastings, NY, USA) version 3.05 and are listed in Table 1.
| Gene Symbol | Forward | Reverse |
|---|---|---|
| PTEN | ACCAGTGGCACTGTTGTTTC | TCCTGTCGTCCTGGTATGAAG |
| DACT1 | CCCCAAATCTGCAGATGTG | TGACGGCATCTAGCTCAGATC |
| β-Actin | TTCGAGCAAGAGATGGCCA | CACAGGACTCCATGCCCAG |
Abbreviation: PTEN, phosphatase and tensin homolog.
3.5. Statistical Analysis
In this study, all experiments, including the MTT assay, LDH cytotoxicity assay, flow cytometry (annexin V/PI), and real-time PCR, were performed in three independent biological replicates. Within each biological replicate, measurements were also conducted in triplicate technical replicates to ensure the reproducibility and reliability of the results. All results were presented as means ± standard deviation (SD). The analysis of the data was carried out using SPSS software (SPSS Inc., Chicago, Illinois, USA) version 22. Group comparisons were executed using Tukey’s test for one-way analysis of variance and t-test. A significance level of P < 0.05 was established for determining differences.
4. Results
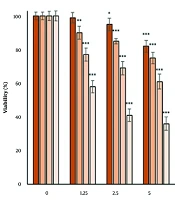
4.1. The Effect of Dioscin on Cell Viability
The effect of dioscin on cell viability was investigated using the MTT test (Figure 1). After 24 hours of treatment, a significant reduction was observed in the concentrations of 2.5, 5, 10, 20, and 40 μM dioscin (P < 0.05). After 48, 72, and 96 hours of treatment, the reduction in cell viability was significant at all concentrations (P < 0.05). The results of the MTT assay showed that dioscin reduced cell viability in a concentration- and time-dependent manner. IC50 values obtained for 24, 48, 72, and 96 hours of treatment were 35.45, 28.36, 10.53, and 1.89 μM, respectively.
The effect of dioscin on the viability of thyroid cancer cells. Cell viability was evaluated after 24, 48, 72 and 96 h of treatment by MTT assay. The cells of the control group received the same volume of medium without drugs (* P < 0.05, ** P < 0.01 and *** P < 0.001 compared to the control).
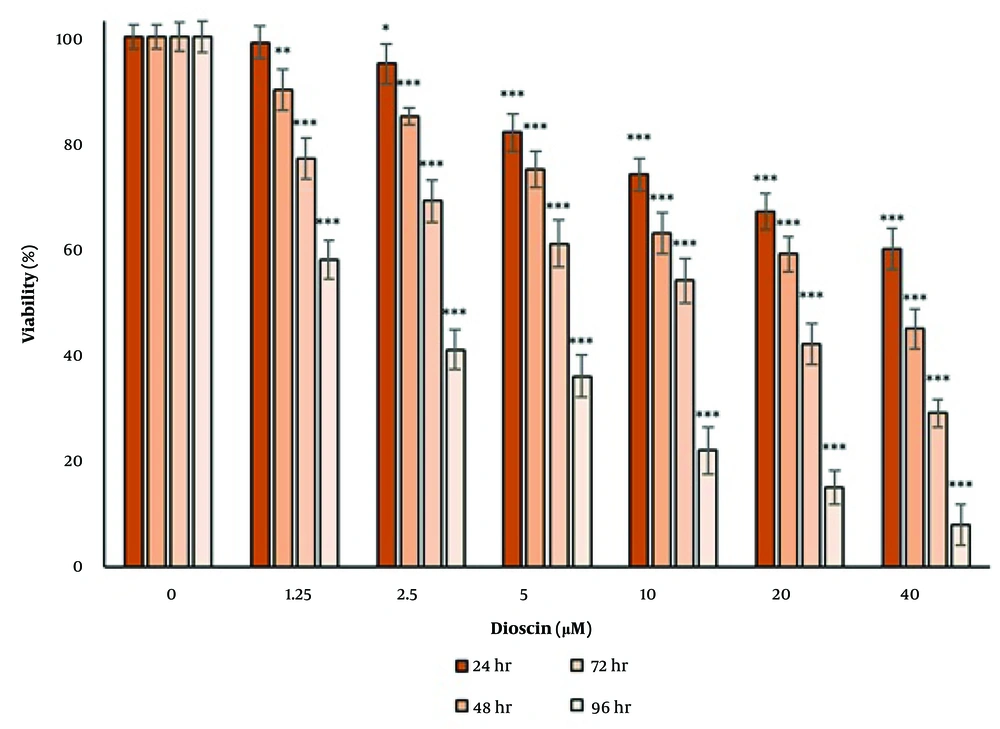
4.2. Cytotoxic Effect of Dioscin
The cytotoxic effect of dioscin was tested by measuring the activity of the lactate dehydrogenase enzyme in the culture medium (Figure 2). After 24 hours of treatment, the cytotoxicity of dioscin was significant at concentrations of 20 and 40 μM (P < 0.05). After 48 hours of treatment, the cytotoxicity of dioscin was significant at concentrations of 10, 20, and 40 μM (P < 0.05). After 72 hours of treatment, the cytotoxicity of dioscin was significant at concentrations of 5, 10, 20, and 40 μM (P < 0.05), and after 96 hours, the cytotoxicity of dioscin was significant at all concentrations (P < 0.05). The results of the lactate dehydrogenase enzyme activity test showed that the cytotoxicity of dioscin was concentration- and time-dependent.
Cytotoxic effect of dioscin on thyroid cancer cells. Cytotoxicity was evaluated after 24, 48, 72 and 96 h of treatment by lactate dehydrogenase enzyme activity. The cells of the control group received the same volume of medium without drugs (** P < 0.01 and *** P < 0.001 compared to the control).
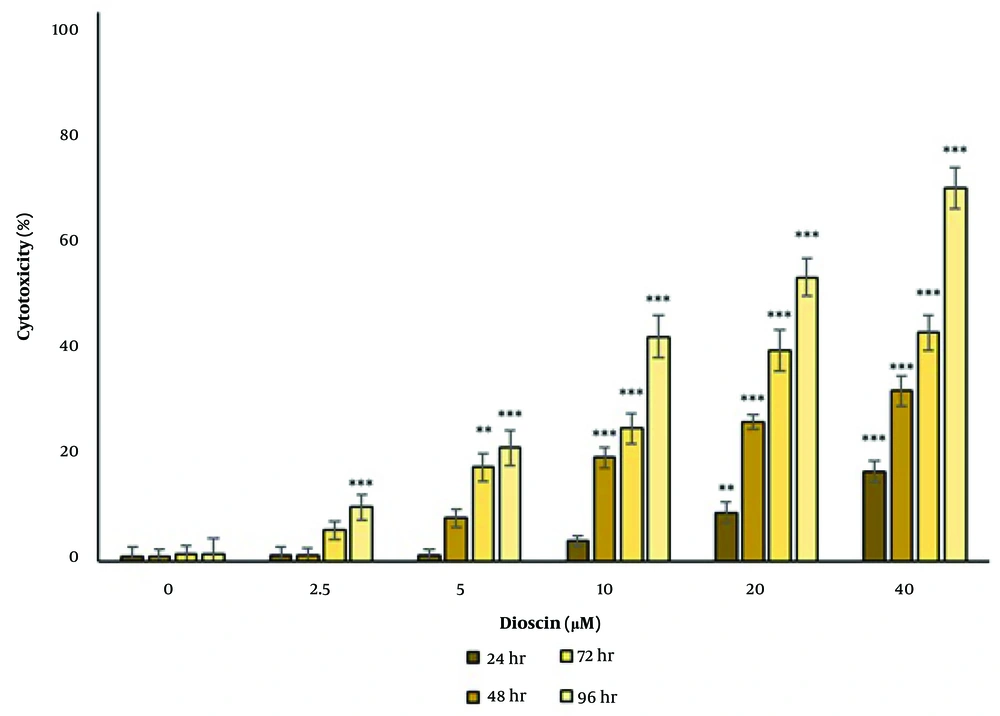
4.3. The Effect of Dioscin on Cell Apoptosis
Examination of apoptosis by the annexin V/FITC method showed that in the control group, 97.3% of cells were alive, 0.75% of cells had early apoptosis, 1.26% had late apoptosis, and 0.74% had necrosis. After 24 hours of treatment with the IC50 concentration, 69.3% of cells were alive, 1.10% had early apoptosis, 26.5% had late apoptosis, and 3.06% had necrosis (Figure 3).
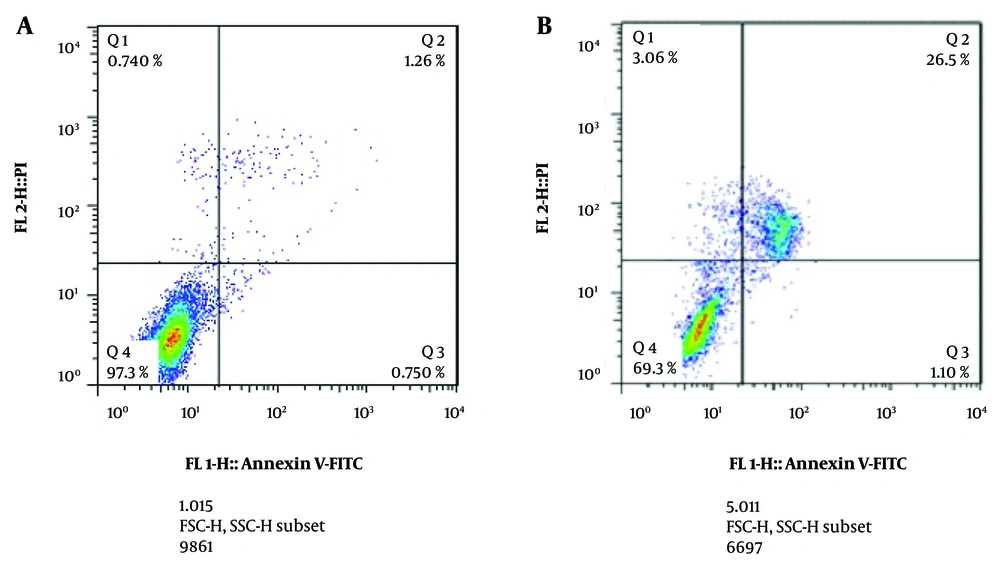
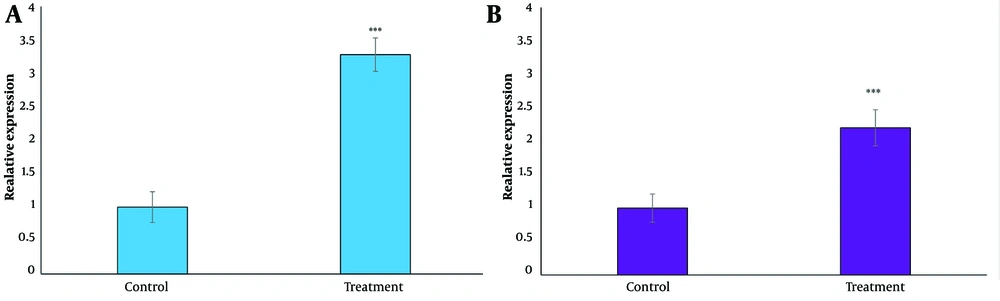
4.4. The Effect of Dioscin on Phosphatase and Tensin Homolog and DACT1 Expression Levels
The effect of dioscin on PTEN and DACT1 expression levels was tested using a real-time PCR test (Figure 4). After 24 hours of treatment with the IC50 concentration, a significant increase in the expression of both genes was observed (P < 0.05).
Effect of dioscin on A, phosphatase and tensin homolog (PTEN) and B, DACT1 gene expression in thyroid cancer cells. Gene expression was analyzed after 24 h of treatment with IC50 concentration by real time PCR test. The cells of the control group received the same volume of medium without drugs (*** P < 0.001 compared to the control).
5. Discussion
In this study, the effect of dioscin on the expression of PTEN and DACT1 genes and apoptosis in thyroid cancer cells was investigated. The results of the MTT test showed that the decrease in cell viability observed during dioscin treatment occurred in a manner that was dependent on both concentration and treatment duration. Inhibitory concentration values obtained for 24, 48, 72, and 96 hours of treatment were 35.45, 28.36, 10.53, and 1.89 μM, respectively. Additionally, the results of the lactate dehydrogenase enzyme activity test showed that the cytotoxicity of dioscin was concentration- and time-dependent. Examination of apoptosis by the annexin V/FITC method showed that dioscin induced apoptosis in cancer cells.
Previous studies have shown that dioscin exhibits anticancer activity against many types of cancers (16). Recently, the range of anticancer efficacy of dioscin has been broadened (17-19). Dioscin promotes apoptosis by activating caspase-9, caspase-3, Bak, Bax, and Bid while also downregulating anti-apoptotic proteins such as Bcl-2, Bcl-xl, cIAP-1, and Mcl-1 in cancer cells. In the context of dioscin-induced apoptosis, the discharge of Ca2+ and the elevated production of endogenous reactive oxygen species (ROS) are prevalent (20-25). Furthermore, the involvement of the PI3K/Akt/mTOR and p38 MAPK and JNK signaling pathways is significant in the anticancer activities attributed to dioscin (26-28).
Additionally, our studies showed that after 24 hours of treatment with the IC50 concentration of dioscin, a significant increase in the expression of PTEN and DACT1 genes was observed. The PTEN is regarded as one of the tumor suppressor components within the human genome, and the loss of function of this gene is observed in certain types of cancer (10, 29). In the current research, it was demonstrated that dioscin reduced cellular proliferation and enhanced apoptosis by elevating PTEN expression. Overexpression of PTEN in ovarian cancer cells inhibits tumor growth and extends survival duration in mice (11). Also, the loss of PTEN in the fallopian tube causes hyperplasia and the formation of ovarian tumor tissue (30). The PTEN plays a major role in the development of ovarian tumors (31). This could account for the notable decrease in cell proliferation seen following the treatment of cells with dioscin, as demonstrated by the MTT assay and the lactate dehydrogenase enzyme activity assay.
DACT1 is an additional tumor suppressor gene that is crucial for the apoptosis and proliferation of cancer cells. It regulates the cell cycle and suppresses cancer cell growth by decreasing nuclear β-catenin levels. Furthermore, this molecule influences the Wnt signaling pathway. Research has demonstrated that abnormal activation of the Wnt/β-catenin pathway in cancer results in β-catenin hyperactivity (32). DACT1 suppresses Wnt signaling and promotes autophagy in cancer (33).
Despite the promising findings, the experiments were limited to a single thyroid cancer cell line (B-CPAP) under in vitro conditions, which do not fully replicate the complexity of in vivo tumor environments. Additionally, the anticancer effects of dioscin were not tested in animal models, limiting our ability to assess pharmacokinetics, systemic toxicity, and therapeutic potential in a whole organism. To address these limitations and further validate the findings, future studies are recommended to utilize in vivo models to evaluate the systemic effects, bioavailability, and toxicity of dioscin.
5.1. Conclusions
The current findings indicate that the application of dioscin on thyroid cancer cells resulted in the suppression of cell growth and proliferation, as well as the induction of apoptosis within these cells. This antiproliferative effect could be attributed to the enhancement of PTEN gene expression, which subsequently inhibits the PI3K/AKT/mTOR signaling pathway, alongside an increase in DACT1 expression that affects the Wnt/β-catenin signaling pathway. Based on these results, dioscin appears to be a potential candidate for the advancement of anticancer therapies.