1. Background
Thyroid cancer is a malignancy of thyroid cells. Recently, there has been an increase in the number of thyroid cancer patients, which may be attributable to the widespread use of imaging for the detection of thyroid nodules. The strategies for thyroid cancer treatment depend on the type and stage of cancer. Despite new treatment methods, this cancer is still associated with a high mortality rate worldwide (1). Studies have shown that some herbal anticancer agents are beneficial in preventing and treating human tumors (2). In the 1950s, scientists began to systematically investigate natural compounds as a source of useful anticancer agents. Recently, it has been suggested that the use of natural products has been the most successful strategy in the discovery of new drugs (3). Andrographis paniculata, an herbaceous plant from the Acanthaceae family, has been used as a kind of medicinal food for a long time (4). Andrographis paniculata is used to reduce the severity and duration of cold symptoms, fever, cough, and sore throat. Many Asian and European researchers have begun to investigate the composition and medicinal properties of this ancient herb. Andrographolide, a labdane diterpenoid, is the major constituent of A. paniculata. It exhibits various medicinal potentials (5, 6).
The PI3K/AKT/mTOR pathway is a crucial signaling pathway involved in the development and progression of thyroid cancer. Dysregulation of this pathway, often through mutations or amplifications of pathway components, can promote tumor cell proliferation, survival, and resistance to treatment. Targeting this pathway with inhibitors is a promising strategy for systemic therapy in advanced thyroid cancer (7). The phosphorylated state of phosphatase and TENsin homolog (PTEN) deleted on chromosome ten operates as a tumor suppressor by downregulating PI3K. The PTEN catalyzes the dephosphorylation of PIP3, which in turn causes the phosphorylation of PI3K/AKT. In cancer cells, PTEN is phosphorylated and is heavily inhibited by the activation of PI3K/AKT (8). Induction of PTEN inhibits the growth of cancer cells and prolongs the survival of mice with disseminated peritoneal tumors (9). The Wnt/β-catenin signaling pathway plays a significant role in the development and progression of thyroid cancer. Aberrant activation of this pathway, often due to mutations or other mechanisms, can lead to uncontrolled cell growth, invasion, and metastasis. The DACT1 is a tumor suppressor gene that inhibits the WNT/β-catenin signaling in cancer cells (10). However, the exact biological functions in cancer pathogenesis are unknown.
2. Objectives
Considering the high prevalence of thyroid cancer in Iran, the increase in the number of patients in recent years, and the need for new treatments, the effect of andrographolide on apoptosis and PTEN and DACT1 mRNA expression levels in thyroid cancer cells was investigated.
3. Methods
3.1. Cell Culture
Thyroid cancer cells (B-CPAP) (Pasteur Institute cell bank, Tehran, Iran) were cultured according to the method described previously (11). Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, Missouri, USA) was utilized to prepare a stock solution of andrographolide (Sigma-Aldrich, Missouri, USA) at a concentration of 10 mg/mL.
3.2. Viability Tests
The impact of andrographolide on cell viability was evaluated using the [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide] (MTT) colorimetric assay. The cells (15 × 103 cells/well) were plated in 96-well microplates and allowed to incubate overnight prior to treatment. Untreated cells served as the control group. The cells were subjected to escalating concentrations (1, 2, 4, 8, 16, 32, 64, and 128 μM) of andrographolide. Subsequently, the MTT assay was conducted as outlined earlier (12). The GraphPad Prism (GraphPad Software, San Diego, California) version 9.1.0 was used to calculate the half-maximal inhibitory concentration (IC50) values.
3.3. Cytotoxicity Assay
The cytotoxic effects of andrographolide were evaluated through the quantification of lactate dehydrogenase (LDH) release into the culture medium. Cells were exposed to concentrations of andrographolide at 1, 2, 4, 8, 16, 32, 64, and 128 µM for durations of 24, 48, 72, and 96 hours. The amount of LDH released into the media was determined using the Cytotoxicity Detection Kit (BCAM, Cambridge, MA, USA), following the protocol provided by the manufacturer (13).
3.4. Apoptosis Assay
The effect of andrographolide on apoptosis was tested as previously described (11, 14). Briefly, the percentage of DNA fragmentation after 24 hours of treatment was determined using the diphenylamine method described by Cohen and Duke, and the absorbance of the samples was determined using a spectrophotometer at 600 nm. Additionally, apoptosis was quantified by the Annexin V-FITC Apoptosis Staining/Detection Kit (ab14085) according to the manufacturer's instructions.
3.5. Molecular Analysis
Gene expression analysis was conducted by real-time PCR as previously described (13). Briefly, RNA was extracted using TRIzol reagent (Thermo Fisher Scientific, USA), and its purity was determined at 260/280 nm. Single-stranded complementary DNA (cDNA) was produced using a cDNA synthesis kit supplied by Vivantis Technologies (Selangor DE, Malaysia) according to the manufacturer’s protocol. Amplification of cDNA was performed using SYBR Green master mix supplied by Thermo Scientific (MA, USA). β-Actin was used as an internal standard gene, against which cDNA was normalized. The relative expression of each target mRNA was calculated based on the comparative Ct (2-∆∆Ct) method. All primer sequences were designed using GeneRunner software (Hastings Software, Hastings, NY, USA) version 3.05 and are listed in Table 1.
| Gene Symbol | Forward | Reverse |
|---|---|---|
| PTEN | ACCAGTGGCACTGTTGTTTC | TCCTGTCGTCCTGGTATGAAG |
| DACT1 | CCCCAAATCTGCAGATGTG | TGACGGCATCTAGCTCAGATC |
| β-Actin | TTCGAGCAAGAGATGGCCA | CACAGGACTCCATGCCCAG |
Abbreviation: PTEN, phosphatase and TENsin homolog.
3.6. Statistical Analysis
The experiments were conducted in triplicate, and all results are presented as means ± standard deviation (SD). Data analysis was carried out using SPSS software (SPSS Inc., Chicago, Illinois, USA) version 22. Group comparisons were executed using one-way analysis of variance and t-test. A significance level of P < 0.05 was established for determining differences.
4. Results
4.1. The Effect of Andrographolide on Thyroid Cancer Cell Viability
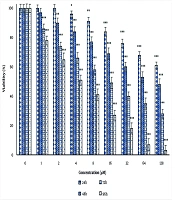
The results of the MTT assay showed that andrographolide significantly reduced cell viability at concentrations of 4, 8, 16, 32, 64, and 128 μM after 24 hours (P < 0.05). It significantly reduced cell viability at 2, 4, 8, 16, 32, 64, and 128 μM after 48 hours (P < 0.05). After 72 and 96 hours, the decrease in cell viability was significant at all concentrations used in this study (P < 0.05) (Figure 1). The IC50 values were 173.97 ± 9.66, 68.82 ± 8.17, 17.36 ± 3.3, and 4.43 ± 0.16 μM for 24, 48, 72, and 96 hours, respectively.
The effect of andrographolide on the viability of thyroid cancer cells. Cell viability was evaluated after 24, 48, 72 and 96 h of treatment by [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide] (MTT) assay. The cells of the control group received the same volume of medium without drugs (*P < 0.05, **P < 0.01, and ***P < 0.001 compared to the control).
4.2. Cytotoxic Effect of Andrographolide on Thyroid Cancer Cells
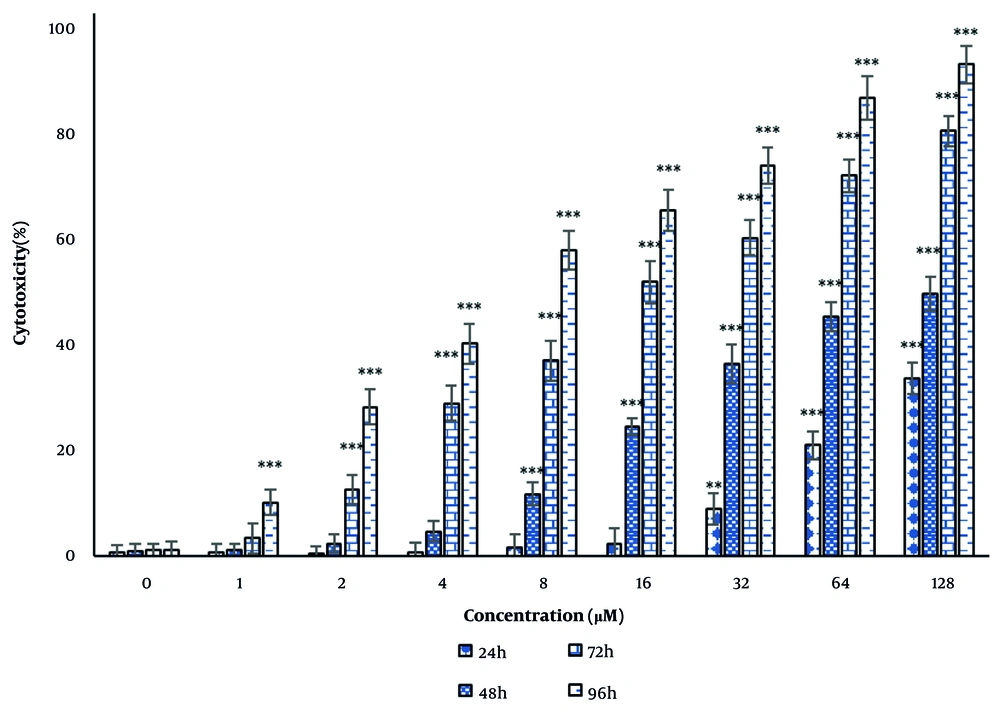
The results of the LDH activity assay showed that andrographolide toxicity was significant at concentrations of 32, 64, and 128 μM (P < 0.05) after 24 hours. After 48 hours, this toxicity was significant at concentrations of 8, 16, 32, 64, and 128 μM (P < 0.05). After 72 hours, the toxicity was significant at concentrations of 2, 4, 8, 16, 32, 64, and 128 μM (P < 0.05). After 96 hours, the cytotoxicity was significant at all concentrations used in this study (P < 0.05) (Figure 2).
Cytotoxic effect of andrographolide on thyroid cancer cells. Cytotoxicity was evaluated after 24, 48, 72 and 96 h of treatment by lactate dehydrogenase (LDH) enzyme activity. The cells of the control group received the same volume of medium without drugs (**P < 0.01, and ***P < 0.001 compared to the control).
4.3. The Effect of Andrographolide on Thyroid Cancer Cell Apoptosis
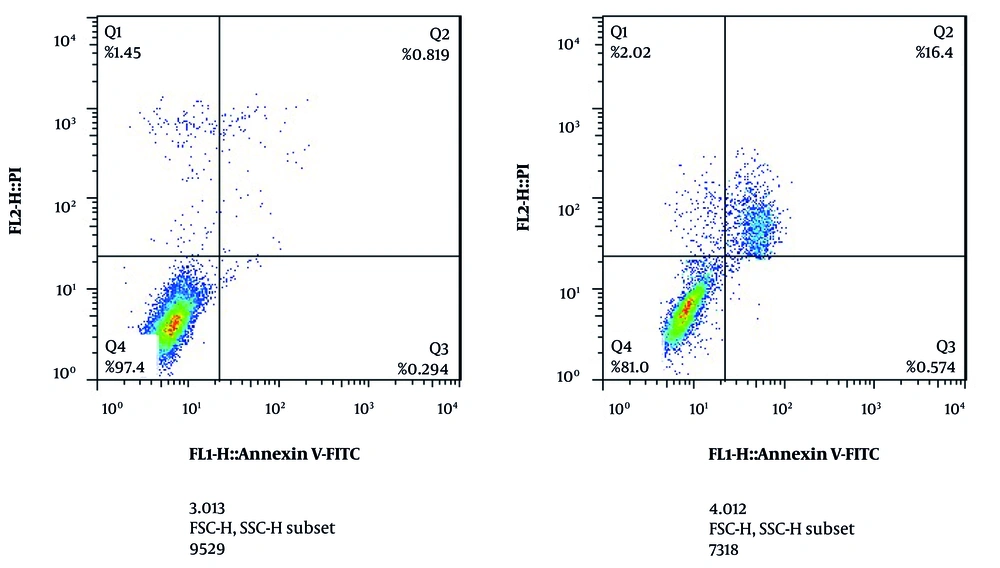
Annexin V/FITC staining showed that in the control group, 97.40% of cells were alive, 0.30% of cells had early apoptosis, 0.82% had late apoptosis, and 1.45% had necrosis. After 24 hours of treatment with the IC50 concentration, 81.00% of cells were alive, 0.58% had early apoptosis, 16.40% had late apoptosis, and 2.02% had necrosis (Figure 3).
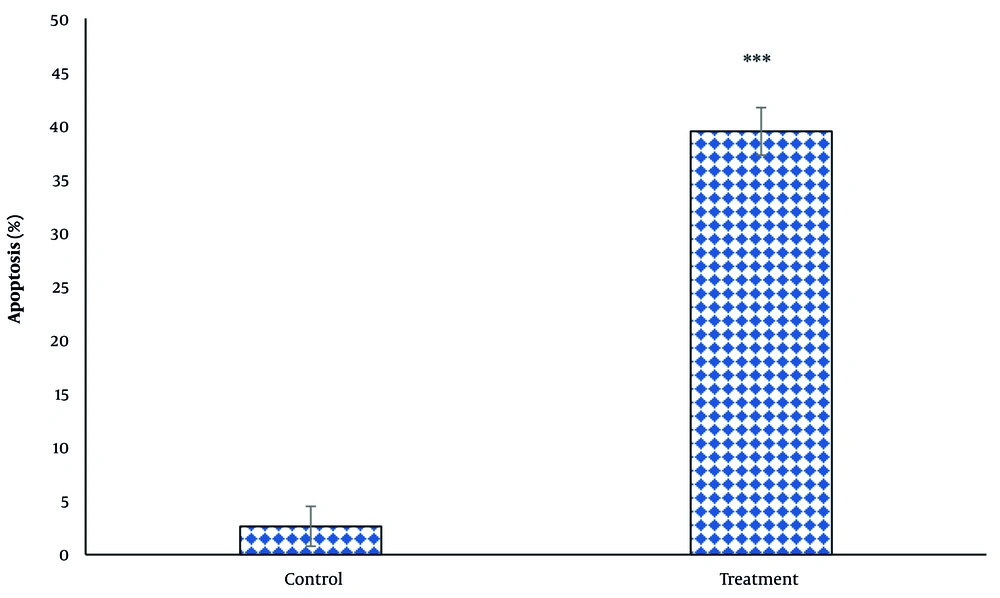
The data from apoptosis measurement using the diphenylamine method showed that after 24 hours of treatment with the IC50 concentration, the amount of apoptosis in thyroid cancer cells increased by 14.95 times. This increase is significant compared to the control group (P < 0.05) (Figure 4).
4.4. The Effect of Andrographolide on Phosphatase and TENsin Homolog and DACT1 Expression in Thyroid Cancer Cells
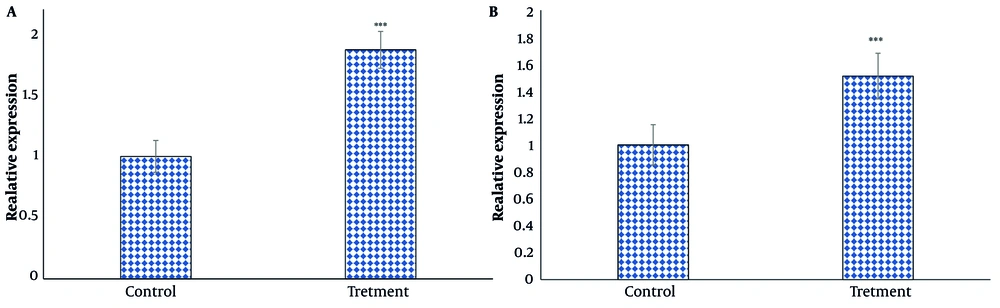
Gene expression analysis showed that andrographolide significantly increased the expression of PTEN and DACT1 genes by 1.87- and 1.51-fold, respectively, in thyroid cancer cells after 24 hours of treatment (P < 0.05) (Figure 5A and B).
Effect of andrographolide on A, phosphatase and TENsin homolog (PTEN); and B, DACT1 gene expression in thyroid cancer cells. Gene expression was analyzed after 24 h of treatment with IC50 concentration by real time PCR test. The cells of the control group received the same volume of medium without drugs (***P < 0.001 compared to the control).
5. Discussion
In recent years, due to the side effects of chemotherapy drugs, people have preferred to use natural herbal products for cancer treatment. Medicinal herbs are traditionally used to treat many diseases. The results of scientific investigations on medicinal plants for the treatment of diseases, including cancer, are promising and have shown that plants can reduce the toxicity of drugs due to their antioxidant properties (15). Studying and investigating agents of natural origin, such as compounds obtained from plants, is one of the most important goals of research in cancer treatments. In this study, the effect of andrographolide, a herbal bicyclic diterpene compound, on viability, apoptosis, and the expression of genes involved in the PI3K/AKT/mTOR and Wnt/β-catenin pathways in a thyroid cancer cell line was investigated. The results showed that treatment with different concentrations of andrographolide decreased cancer cell viability in a concentration- and time-dependent manner. The IC50 values were 173.97 ± 9.66, 68.82 ± 8.17, 17.36 ± 3.3, and 4.43 ± 0.16 μM for 24, 48, 72, and 96 hours, respectively. This decrease in survival was accompanied by damage to the cell membrane and the release of the LDH enzyme into the culture medium. Molecular analysis results showed that andrographolide significantly increased the expression level of PTEN by 1.87 times and DACT1 by 1.51 times.
Previous studies have shown that andrographolide treatment inhibits the proliferation of various types of cancer cells. It exerts direct anticancer activity by inducing the cell cycle inhibitor protein p27 and reducing the expression of cyclin-dependent kinase 4 (CDK4), thereby arresting the cell cycle in the G0/G1 phase. Andrographolide exhibits indirect anticancer properties by enhancing the cytotoxic effects of lymphocytes on cancer cells. Therefore, it can be regarded as a significant compound with both anticancer and immunomodulatory effects, making it a potential therapeutic agent for cancer (16). Specifically, in colon cancer, andrographolide reduces cell viability. Furthermore, andrographolide promotes apoptosis, which correlates with elevated intracellular ROS levels and the disruption of mitochondrial membrane potential (17). In melanoma cancer, andrographolide potentially inhibits cell proliferation and induces apoptosis (18). An abundance of in vitro studies has shown that targeting apoptosis by herbal compounds in cancer cells is possible. However, they must undergo critical trials before they can be safely used in humans (19).
Additionally, data indicated that the expression levels of PTEN and DACT1 genes in thyroid cancer cells increased significantly after treatment with andrographolide. The PTEN serves as a crucial tumor suppressor, and its loss of function has been shown in some cancers (8, 20). The data from this study showed that andrographolide decreased cell growth and increased apoptosis by increasing PTEN expression. The PTEN plays a major role in the formation and development of ovarian cancer, and its overexpression can suppress tumor growth in vivo (9, 21, 22). This may explain the mechanism of andrographolide cytotoxicity observed after the treatment of cells in our study. The DACT1 functions as a tumor suppressor gene and plays a crucial role in regulating apoptosis and the proliferation of cancer cells by lowering nuclear β-catenin levels. This molecule also influences the Wnt/β-catenin signaling pathway. Research indicates that abnormal activation of the Wnt/β-catenin pathway in cancer results in β-catenin hyperactivity (23). The DACT1 inhibits the Wnt/β-catenin pathway and activates autophagy in cancer (24).
5.1. Conclusions
The present results showed that andrographolide reduced cell viability and induced apoptosis in thyroid cancer. Andrographolide may affect the PI3K/AKT/mTOR pathway through an increase in PTEN expression and the Wnt/β-catenin signaling pathway through an increase in DACT1 expression.
![The effect of andrographolide on the viability of thyroid cancer cells. Cell viability was evaluated after 24, 48, 72 and 96 h of treatment by [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide] (MTT) assay. The cells of the control group received the same volume of medium without drugs (*P < 0.05, **P < 0.01, and ***P < 0.001 compared to the control). The effect of andrographolide on the viability of thyroid cancer cells. Cell viability was evaluated after 24, 48, 72 and 96 h of treatment by [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide] (MTT) assay. The cells of the control group received the same volume of medium without drugs (*P < 0.05, **P < 0.01, and ***P < 0.001 compared to the control).](https://services.brieflands.com/cdn/serve/3170c/bc51880bbc59a937ba78fcb32dba5b9d98c7111c/jcrps-14-2-161678-i001-preview.webp)