1. Background
The coronavirus disease (COVID-19) was a global pandemic that began in Wuhan, China, in 2019. The disease affected many patients and members of the community (1-4). Despite the impact of COVID-19 on patients' health and the worsening of clinical symptoms, it also caused an impairment of life, daily activities, and lifestyle changes (5-7). Given the involvement of the airways and lungs and the activation of the inflammatory pathway by COVID-19, immunocompromised patients are among the most susceptible to COVID-19 (6, 8). Rheumatoid arthritis (RA) is a type of autoimmune disease in which the body's organs and tissues are destroyed by the immune system (9). The RA has a broad spectrum of diseases with different clinical symptoms. The RA patients were susceptible to COVID-19 because their immune system is suppressed and cannot effectively fight COVID-19 (10). Therefore, the mortality rate of these patients due to COVID-19 has increased. Although COVID-19 was an emerging disease for which there was initially no specific treatment, a vaccine against COVID-19 was developed over time, which improved the clinical symptoms of patients to a certain extent (11, 12). There is evidence that patients with COVID-19 who had been vaccinated experienced an increased recurrence of RA (10). However, the evidence and data on these topics were limited. Therefore, only a few studies have investigated the association between RA recurrence and one type of vaccine.
2. Objectives
In this study, we investigated the association between RA recurrence and the number of injections and the brand of each vaccine.
3. Methods
3.1. Design Study and Patient Selection
This is a cross-sectional study. The patients participating in this study are those with RA who have undergone COVID-19 vaccination. Ninety-eight patients (convenience sampling) were analyzed in this study. They were selected from the patients admitted to the Rheumatology Department of Al-Zahra Hospital, Isfahan, between 2020 and 2021. It is important to note that two patients were excluded from the study because they did not meet the inclusion criteria. The patients' RA disease was under control and had a disease activity score-28 (DAS-28 < 2.6). All patients were examined by a rheumatologist prior to participation in the study. Informed consent was obtained from all patients before participation in the study. The DAS28 can be calculated using the following formula (mdcal.com):
3.2. Inclusion and Exclusion Criteria
Inclusion criteria included patients with RA and those undergoing vaccination. Exclusion criteria included a previous history of infection and patients without informed consent to participate in the study.
3.3. Data Collection
After the patients were selected, their demographic data were collected from their records. In addition, the vaccine brands, injection doses, and outcomes related to recovery or recurrence of the disease were recorded in the corresponding checklist. It is important to note that the drug treatment of the patients was carried out according to the doctor's orders, and there were no changes. The patients' data were collected between 2020 and 2022, as recorded in their notes. All patients had also undergone vaccination in all three doses. The type of vaccination administered to the patients was the same for each dose.
3.4. Statistical Analysis
Data were expressed as number (percentage) and mean ± standard deviation (SD). Statistical analyses were performed using SPSS software version 23 (IBM Corp., Armonk, NY, USA). A P-value < 0.05 was considered statistically significant. Continuous variables were tested for normality using the Shapiro-Wilk test. Since most continuous variables were not normally distributed, they were presented as median [interquartile range (IQR)]. Categorical variables were presented as number (percentage). Comparisons between categorical variables were conducted using Fisher’s exact test. For comparisons between two related groups of continuous variables, the Wilcoxon signed-rank test was applied, and for comparisons among more than two groups, the Kruskal-Wallis test was used. The significance level (α) was set at 0.05 (Z = 1.96) and the study power (1 - β) at 80% (Z = 0.84). The event rates (improvement or worsening of DAS-28) were based on preliminary data: In the first dose group receiving the Sinopharm vaccine, 30% of patients showed improvement and 25% showed worsening of DAS-28; in the Sputnik vaccine group, the corresponding rates were 20% and 15%; and in the AstraZeneca vaccine group, 15% showed improvement and 20% worsening. The sample size was calculated using the following formula:
Based on these calculations, the required sample size was estimated to be approximately 100 participants per vaccine group.
4. Results
Eleven patients were immunized only once. Thirty patients were vaccinated twice, 54 patients three times, and only 3 patients four times. Most patients were immunized with vaccines from Sinopharm and AstraZeneca.
4.1. Descriptive Analysis of Data
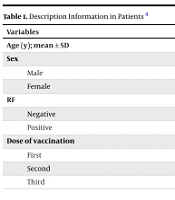
The data analysis showed that the mean age of the patients was 54.55 ± 11.58 years. Seventy-eight patients (79.6%) were female, and 20 (20.4%) were male. Regarding RF, 65 patients (66.3%) were positive, and 33 (33.7%) were negative. With regard to the injection dose, the results showed that 55 patients (56.1%) received dose III, and 12 patients (12.2%) received dose I (Table 1).
| Variables | Values |
|---|---|
| Age (y); mean ± SD | 54.55 ± 11.58 |
| Sex | |
| Male | 20 (20.4) |
| Female | 78 (79.6) |
| RF | |
| Negative | 33 (33.7) |
| Positive | 65 (66.3) |
| Dose of vaccination | |
| First | 12 (12.2) |
| Second | 31 (31.6) |
| Third | 55 (56.1) |
Abbreviations: SD, standard deviation; RF, rheumatoid factor.
a Values are expressed as No. (%) unless otherwise indicated.
4.2. Relation Between Vaccination Dose and Disease Activity Score-28
Based on the data analysis, the results showed changes in DAS-28 scores before and after each vaccine dose. In dose I, the mean DAS-28 increased from 1.66 ± 0.31 (median = 1.7, IQR = 0.9) to 1.79 ± 0.63 (median = 1.6, IQR = 0.3), which was statistically significant (P = 0.02). For dose II, the DAS-28 score changed from 1.66 ± 0.30 (median = 1.5, IQR = 0.8) to 1.72 ± 0.62 (median = 1.6, IQR = not reported), without reaching statistical significance (P = 0.31). Similarly, dose III showed a change from 1.65 ± 0.31 (median = 1.5, IQR = 0.6) to 1.77 ± 0.77 (median = 1.5, IQR = 0.5), which was not statistically significant (P = 0.24). All P-values were calculated using the Wilcoxon signed-rank test for paired comparisons. These results suggest a small but statistically significant increase in DAS-28 following the first vaccine dose, whereas subsequent doses did not result in significant changes (Table 2).
| Doses | DAS-28 | P-Value a | |
|---|---|---|---|
| Before | After | ||
| Dose I | 0.02 | ||
| Mean ± SD | 1.66 ± 0.31 | 1.79 ± 0.63 | |
| Median [IQR] | 1.7 [0.9] | 1.6 [0.3] | |
| Dose II | 0.31 | ||
| Mean ± SD | 1.66 ± 0.30 | 1.72 ± 0.62 | |
| Median [IQR] | 1.5 [0.8] | 1.6 [-] | |
| Dose III | 0.24 | ||
| Mean ± SD | 1.65 ± 0.31 | 1.77 ± 0.77 | |
| Median [IQR] | 1.5 [0.6] | 1.5 [0.5] | |
Abbreviations: DAS-28, disease activity score-28; SD, standard deviation; IQR, interquartile range.
a Calculated by Wilcoxon signed-rank test.
4.3. Relation Between Disease Activity Score-28 Before and After Vaccination Based on Type of Vaccine
The results showed that at dose I, the Sinopharm vaccine had a higher number of patients with improvement and worsening of DAS-28 scores compared to other vaccines. In contrast, none of the patients who received the Sputnik vaccine showed a higher number of patients with DAS-28 improvement and deterioration (P = 0.55). In dose II, the Sinopharm vaccine had a higher number of patients with DAS-28 improvement and worsening compared to the other vaccines. In contrast, none of the patients who received the Barekat vaccine had a higher number of patients with DAS-28 improvement and worsening (P = 0.51). At dose III, only one patient who received the Barekat vaccine had an improvement in DAS-28. In contrast, five patients who received the Sinopharm vaccine had a worsening of DAS-28 compared to the pre-injection period (P = 0.21, Table 3).
| Doses; Vaccine | DAS-28 | P-Value b | ||
|---|---|---|---|---|
| Better | Equal | Worse | ||
| Dose I | 0.55 | |||
| Barekat | 1 (14.3) | 5 (6.5) | 1 (7.1) | |
| Sinopharm | 5 (71.4) | 57 (74) | 12 (85.7) | |
| AstraZeneca | 1 (14.3) | 13 (16.9) | 1 (7.1) | |
| Sputnik | 0 (0) | 2 (2.6) | 0 (0) | |
| Dose II | 0.51 | |||
| Barekat | 0 (0) | 9 (10.3) | 0 (0) | |
| Sinopharm | 5 (100) | 66 (75.9) | 5 (83.3) | |
| AstraZeneca | 2 (28.6) | 12 (13.8) | 1 (16.7) | |
| Dose III | 0.21 | |||
| Barekat | 1 (100) | 9 (10.2) | 0 (0) | |
| Sinopharm | 0 (0) | 61 (69.3) | 5 (55.6) | |
| Pastocovac | 0 (0) | 5 (5.7) | 1 (11.1) | |
| AstraZeneca | 0 (0) | 13 (14.8) | 3 (33.3) | |
Abbreviation: DAS-28, disease activity score-28.
a Values are expressed as No. (%).
b Calculated by Fisher’s exact test.
5. Discussion
The COVID-19 was one of the pandemics that, despite the social problems, affected many healthy and sick individuals. Due to the novelty of the virus, there was no definitive and known treatment for it. Gradually, with the understanding of the virus's structure and its pathogenesis, vaccines were designed against the virus (13). Although various vaccines were developed against COVID-19, only a few of them had adequate efficacy for proper immunogenicity against the virus. Vaccination in patients, despite the improvement of clinical conditions, in some cases caused a flare-up of the disease in RA patients (14).
In the present study, the results showed that the mean DAS-28 increased after vaccination in all three doses compared to before. However, these differences were only significant in dose I (P = 0.03). Sinkovec Savsek et al. showed that the incidence of relapse in children with RA was higher after infection with COVID-19 compared to their vaccination (15). The results of this study were consistent with the present study; however, the present study showed that in some patients, disease severity according to DAS-28 increased after vaccination. Bixio et al. demonstrated that a small number of RA patients who had recovered and were vaccinated with BNT162b2 (BioNTech-Pfizer) experienced a disease flare. Consistent with these results, a small number of patients in the present study experienced a disease flare after vaccination (16). Safary et al. showed that RA patients who were vaccinated experienced a small number of exacerbations and disease flares. This exacerbation was caused by external factors and was not related to the vaccination of the patients (17). Geng et al. demonstrated that there was no association between the vaccination of patients and disease flares. Their results indicated that patients whose disease was under control or had a low level of disease activity did not experience any disease flares after vaccination (18). A meta-analysis study showed that there was no association between COVID-19 vaccination and RA recurrence; on the contrary, vaccination may have a protective role against disease severity (19). Fan et al. found that relapse in RA patients occurs only rarely after vaccination; however, in most cases, clinical improvement is observed (20). Qian et al. reported that vaccination of COVID-19 patients had no effect on the incidence of flares of RA (21). In the present study, the results showed that patients who were vaccinated with Sinopharm had more severe clinical symptoms in all three doses compared to other vaccines; however, there was no statistically significant relationship between them.
In the study by Safary et al., clinical symptoms were more severe in patients vaccinated with AstraZeneca compared to other types of vaccines. These results were not consistent with the present study (17). In another study, Delkash et al. showed that patients receiving disease-modifying antirheumatic drugs (DMARDs) and vaccinated with Sinopharm had a low incidence of disease flare, moderate severity, and no hospitalization (22). In line with the present study, Sahebari et al. demonstrated that about 3% of RA patients who underwent COVID-19 vaccination experienced a disease flare. The results indicated that the frequency of disease flare was higher in patients receiving Sinopharm than in those receiving other types of vaccines, as observed in the present study (23). The strengths of this study were the large sample size and the exclusion of patients with poor compliance. By omitting these patients, we eliminated the effect of data from patients with low control situations that could adversely bias the results. Additionally, all patients were examined by one person during the study, ensuring that the examination process was consistent for all patients.
A limitation of this study was the fact that the majority of Iranians, including patients with RA, were vaccinated with the Sinopharm vaccine, and vaccines such as Pfizer or Johnson & Johnson were not routinely used in Iran, so we could not evaluate the effect of such vaccines. Another limitation of our study was the lack of long-term follow-up; in other words, we only evaluated the patients two weeks after vaccination. Therefore, other studies should be conducted to follow up with patients for a longer period. Another limitation was the small sample size of the study participants. Additionally, the patients were selected from a single treatment center.
5.1. Conclusions
In general, based on the results, it can be said that there was no relationship between the type of vaccination and DAS-28. In other words, the vaccination of patients had no effect on the improvement or deterioration of the clinical condition of patients based on DAS-28. It is important to note that the brands used in this study included Barekat, Sinopharm, Pastocovac, and AstraZeneca. Therefore, it is better to investigate other brands of vaccines in future studies.