1. Background
Oral squamous cell carcinoma (OSCC) is the most common form of head and neck cancer, posing a major public health issue worldwide due to its aggressive behavior and the severe functional impairment it causes (1). In Iran, specifically in the southwestern province of Khuzestan, the incidence of oral and oropharyngeal cancers has been a growing concern, with studies indicating distinct regional patterns that demand localized investigation (2, 3). Recent data from Ahvaz, the capital of Khuzestan, confirm this trend, underscoring the need for continued research into the multifaceted burden of this disease on the local population (4). While survival outcomes and clinical prognostic factors have been explored in this region (5), the complex interplay between the physical symptoms and psychosocial dimensions of the OSCC experience remains less elucidated.
The progression of OSCC is notoriously severe, with pain standing out as a dominant and often difficult-to-manage symptom. This cancer-related pain stems from multiple sources, such as the tumor invading nerve-rich orofacial tissues, side effects from treatments like radiation and chemotherapy, and subsequent infections (6, 7). Importantly, this pain is not just a physical sensation; it frequently includes a significant neuropathic element, which adds to its intensity and complicates treatment with standard painkillers (8). Uncontrolled pain is a primary determinant of suffering, leading to functional impairments in mastication, swallowing, and speech, negatively affecting nutritional health and limiting a patient's ability to engage in social activities (9, 10).
A particularly disruptive, yet often overlooked, consequence for OSCC patients is a severe decline in sleep quality. A strong consensus in cancer research confirms a direct link between chronic pain and sleep problems, creating a self-reinforcing and damaging cycle (11, 12). Pain can prevent patients from falling asleep or staying asleep, and conversely, the sleep deprivation that results can heighten a person's sensitivity to pain, a condition known as hyperalgesia (13, 14). In head and neck cancer patients, factors such as airway obstruction, xerostomia, nocturnal drooling, and psychological distress further compound these sleep disruptions (15, 16). Poor sleep quality is not a mere inconvenience; it is independently associated with worsened quality of life (17), increased depression and anxiety (18), impaired cognitive function (19), and may even negatively influence immune function and treatment outcomes (20).
While the pain-sleep relationship is recognized in broader oncology, its specific dynamics within the unique context of OSCC — particularly in specific cultural and geographical settings — are not fully characterized. Furthermore, this relationship does not exist in a vacuum; it is profoundly modulated by a range of socio-demographic factors. Socio-economic status (SES), for instance, is a well-established determinant of health outcomes in cancer, influencing stage at diagnosis, access to timely and adequate pain management, and availability of supportive care resources (21, 22). Educational level can affect health literacy and a patient's ability to comprehend and manage their symptoms (23). Marital status may influence the availability of social support, which can buffer against the psychological distress that exacerbates both pain and sleep problems (24, 25). Cultural beliefs and practices regarding pain expression and sleep hygiene can also vary significantly, potentially influencing reported outcomes (26).
In the specific context of Ahvaz, Iran, understanding these interrelationships is crucial. The local population faces unique socio-economic challenges and cultural norms that may distinctively shape the experience of OSCC. To date, no study in this region has simultaneously examined the triad of sleep quality, pain intensity, and key socio-demographic variables in a single cohort of OSCC patients.
2. Objectives
This study aimed to bridge this critical knowledge gap. We hypothesize that in patients with OSCC in Ahvaz, poor sleep quality is significantly associated with higher pain intensity, and that this relationship is moderated by specific socio-demographic factors such as SES, education, and marital status. We aimed to explore the interplay of sleep quality, pain, and socio-demographic factors in patients with OSCC.
3. Methods
3.1. Study Design and Setting
This investigation employed a cross-sectional, analytical study design to elucidate the complex interplay between sleep quality, pain intensity, and socio-demographic factors in patients with OSCC. The study was conducted at the Cancer Registry Center in Ahvaz city, a public cancer facility in southwest Iran. Participants were invited to Golestan Hospital. These centers are the principal hubs for the diagnosis and management of cancers in the Khuzestan province. The researcher contacted individuals to obtain informed consent and explain the study's objectives. Data collection was carried out over a defined 5-month period (December 2024 to May 2025) to ensure a contemporary snapshot of the patient experience.
3.2. Participants and Sampling: A Census Approach
The study population comprised all eligible adult patients with a histopathologically confirmed diagnosis of OSCC attending the aforementioned centers during the data collection period.
1. Inclusion criteria:
- Age 18 years or older
- A new or existing diagnosis of primary OSCC, regardless of treatment phase (newly diagnosed, undergoing active treatment, or in follow-up)
- Sufficient cognitive and communicative ability to comprehend and respond to the Persian-language questionnaires
- Willingness to participate by providing written informed consent
2. Exclusion criteria:
- Presence of known severe cognitive impairment (e.g., dementia) or major psychiatric disorders that could impede reliable self-reporting
- A pre-existing, diagnosed chronic sleep disorder unrelated to cancer (e.g., obstructive sleep apnea, narcolepsy)
- Inability to communicate in Persian
Given the relatively finite and accessible nature of the OSCC patient population at these tertiary centers, a census sampling method was employed. This approach aimed to recruit the entire universe of eligible patients presenting during the study window, rather than a random subset. The final sample size was determined by the total number of patients who met the criteria and consented to participate within this timeframe, which was anticipated to be 75 patients. This figure is consistent with the patient flow documented in recent local studies on OSCC and is deemed sufficient for robust correlational and multivariate analyses in a focused, single-disease cohort.
3.3. Data Collection Tools and Measures
Data were collected through a structured interview conducted by a trained student of dentistry and a subsequent review of the patient's medical records. The assessment battery included:
3.3.1. Socio-demographic and Clinical Characteristics Sheet
A researcher-designed proforma was used to collect key variables. These included:
- Socio-demographics: Age, gender, educational status, occupation, marital status, monthly household income, and place of residence (urban/rural)
- Clinical characteristics: Data extracted from medical records included tumor site (e.g., tongue, buccal mucosa, gingiva)
3.3.2. Pittsburgh Sleep Quality Index
Sleep quality over the preceding month was assessed using the validated Persian version of the Pittsburgh Sleep Quality Index (PSQI) (27). This 19-item instrument generates seven component scores: Subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. The sum of these components yields a global score ranging from 0 to 21, with a score > 5 demonstrating high sensitivity and specificity for distinguishing "poor" from "good" sleepers (28). The PSQI has been extensively validated and is a gold-standard measure in oncological populations, including those with head and neck cancer.
3.3.3. Visual Analogue Scale for Pain
Pain intensity was measured using a 100-mm horizontal Visual Analogue Scale (VAS). The scale was anchored with the phrases "no pain" (0 mm) on the left and "worst pain imaginable" (100 mm) on the right. Patients were instructed to mark a vertical line on the scale that best represented their average pain intensity over the past week. The score was determined by measuring the distance from the left anchor to the patient's mark in millimeters. The VAS is a highly reliable and sensitive tool for measuring subjective pain experiences in cancer patients (29). Its simplicity makes it particularly suitable for populations with varying literacy levels.
3.4. Data Collection Procedure
Ethical approval was secured from the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS.REC.1403.485). Upon identification by their treating physician, eligible patients were approached in a private consultation room. The study's aims, procedures, and confidentiality assurances were thoroughly explained. Written informed consent was obtained from all participants. The research assistant then administered the PSQI and VAS in a single session. The socio-demographic and clinical data sheet was completed partly through patient interviews and partly through medical chart reviews immediately afterward.
3.5. Statistical Analysis
Data analysis was performed using SPSS Statistics software (Version 22.0, IBM Corp., Armonk, NY). Descriptive statistics were computed for all variables: Frequencies and percentages for categorical data, and mean ± standard deviation for continuous data, based on their distribution normality (assessed via the Kolmogorov-Smirnov test).
3.5.1. Bivariate Analyses
The relationship between the primary outcome variable (PSQI global score) and pain (VAS score) was examined using Spearman's rank correlation for non-parametric data. Associations between PSQI scores and categorical socio-demographic factors (e.g., gender, education level) were tested using non-parametric equivalents, the Mann-Whitney U and Kruskal-Wallis H tests.
3.6. Ethical Considerations
This study was conducted in strict adherence to the ethical principles of the Declaration of Helsinki. Participant anonymity and confidentiality were guaranteed. Participants were informed that their participation was entirely voluntary and that withdrawal from the study at any point would not compromise their medical care in any way.
4. Results
4.1. Sample Characteristics
The study included 75 participants with a mean age of 58.4 years. The majority were male (60%), married (70.7%), and covered by social security insurance (53.3%). Most resided in urban areas (77.3%) and had an education level of an associate degree or higher (56%). Slightly more than half were retired (56%), and self-reported SES was predominantly medium (50.7%). A significant majority had more than three children (74.7%). In terms of clinical characteristics, 57.3% had been diagnosed for less than one year, and 72% had no significant comorbidities. The detailed demographic and clinical profile of the participants is presented in Table 1.
| Variables | No. (%) |
|---|---|
| Gender | |
| Male | 45 (60) |
| Female | 30 (40) |
| Marital status | |
| Single | 22 (29.3) |
| Married | 53 (70.7) |
| Health insurance | |
| Social security | 40 (53.3) |
| Rural insurance | 6 (8) |
| Health insurance | 20 (26.7) |
| Other | 9 (12) |
| Residence | |
| Urban | 58 (77.3) |
| Rural | 17 (22.7) |
| Education | |
| High school diploma or less | 33 (44) |
| Associate degree or higher | 42 (56) |
| Employment status | |
| Employed | 40 (55) |
| Retired | 35 (45) |
| SES | |
| Medium | 38 (50.7) |
| Low | 26 (34.7) |
| High | 11 (14.7) |
| Number of children | |
| > 3 | 19 (25.3) |
| 3 < | 56 (74.4) |
| Time since diagnosis (y) | |
| Less than 1 | 43 (57.3) |
| 1 and more | 32 (42.7) |
| Comorbidities | |
| None | 54 (72) |
| Cardiovascular disease | 8 (10.7) |
| Diabetes | 6 (8) |
| Hypertension | 4 (5.3) |
| Thyroid disease | 2 (2.7) |
| Renal disease | 1 (1.3) |
Abbreviation: SES, socio-economic status.
4.2. Sleep Quality, Pain, and Their Interrelationship
The descriptive statistics show that the sample, on average, experienced poor sleep quality, as indicated by a mean ± SD PSQI score of 9.64 ± 3.60, which is substantially above the clinical cut-off of 5. Participants also reported a moderate to severe level of pain, with a mean ± SD VAS score of 6.50 ± 2.10. The core finding is the Spearman correlation coefficient presented in the matrix. The value at the intersection of row 2 and column 1 is 0.68. This indicates a strong, positive, and statistically significant correlation between the global PSQI score and pain intensity (rs = 0.68, P < 0.001). This indicates that as pain levels increased, sleep quality significantly worsened. This central finding is visualized in Table 2.
| Variables | Mean ± SD | 1 | 2 | P-Value |
|---|---|---|---|---|
| PSQI score | 9.64 ± 3.60 | - | < 0.001 | |
| Pain intensity (VAS) | 6.50 ± 2.10 | 0.68 | - |
Abbreviations: SD, standard deviation; PSQI, Pittsburgh Sleep Quality Index; VAS, Visual Analogue Scale.
4.3. Sleep Quality and Components Across Different Age Groups
A statistically significant difference across age groups was found only for "Sleep adequacy" (P = 0.040). An examination of the means shows that the 25 - 34.9 age group reported the highest sense of sleep adequacy (0.75), whereas the oldest group (> 75) reported the lowest (0.00). For all other components, no significant association with age was observed, as shown in Table 3.
| Components | Age Groups (y) | P-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| < 25 | 25 - 34.9 | 35 - 44.9 | 45 - 54.9 | 55 - 64.9 | 65 - 74.9 | > 75 | ||
| Overall self-assessment of sleep quality | 2.33 ± 1.15 | 3.25 ± 0.96 | 2.20 ± 0.84 | 2.76 ± 0.90 | 2.16 ± 1.0 | 2.00 ± 1.03 | 2.00 ± 1.15 | 0.137 |
| Sleep onset delays | 1.67 ± 1.15 | 1.50 ± 1.00 | 1.20 ± 0.45 | 2.12 ± 0.78 | 1.68 ± 0.82 | 1.50 ± 0.83 | 1.43 ± 0.79 | 0.152 |
| Duration of effective sleep | 0.67 ± 1.15 | 1.25 ± 1.50 | 1.20 ± 0.84 | 1.35 ± 0.79 | 1.16 ± 1.12 | 1.35 ± 0.93 | 1.57 ± 1.13 | 0.896 |
| Sleep adequacy | 1.00 ± 1.00 | 0.75 ± 1.50 | 0.20 ± 0.45 | 0.12 ± 0.33 | 0.05 ± 0.23 | 0.15 ± 0.67 | 0.00 ± 0.00 | 0.040 |
| Sleep disturbance | 1.67 ± 1.15 | 2.25 ± 0.50 | 1.40 ± 0.55 | 2.00 ± 0.61 | 1.95 ± 0.85 | 1.50 ± 0.69 | 1.71 ± 0.76 | 0.172 |
| Amount of sedative medication used | 0.00 ± 0.00 | 0.75 ± 0.96 | 0.40 ± 0.55 | 1.00 ± 1.27 | 1.00 ± 1.33 | 0.50 ± 1.10 | 1.14 ± 0.38 | 0.540 |
| Morning performance | 1.00 ± 0.00 | 2.50 ± 0.58 | 1.80 ± 1.10 | 1.76 ± 0.83 | 1.76 ± 0.83 | 1.60 ± 0.88 | 1.14 ± 0.38 | - |
| Sleep quality | 8.33 ± 3.21 | 12.25 ± 3.86 | 8.40 ± 2.88 | 11.12 ± 3.22 | 9.84 ± 3.78 | 8.60 ± 3.66 | 8.43 ± 3.46 | 0.184 |
a Values are expressed as mean ± SD.
The analysis of associations between PSQI components and a wide range of demographic variables yielded several key findings, with most factors showing no significant association.
4.3.1. Employment Status
Employed patients reported significantly worse subjective sleep quality (2.55 ± 1.01 vs. 2.05 ± 0.99; Z = -2.100, P = 0.036) and greater daytime dysfunction (1.89 ± 0.89 vs. 1.49 ± 0.77; Z = -2.077, P = 0.038) compared to retired patients. The PSQI score was also higher in employed individuals, though this difference was not statistically significant (10.18 ± 3.28 vs. 9.08 ± 3.86; P = 0.119).
4.3.2. Other Demographic Factors
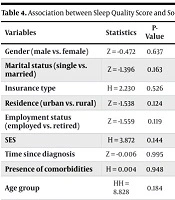
No statistically significant associations were found between any PSQI component and the following variables: Gender, marital status, type of health insurance, place of residence, SES, time since diagnosis, or the presence of specific comorbidities (all P-values > 0.05). The detailed results of these analyses are consolidated into a single, comprehensive table (Table 4) to provide a clear overview.
| Variables | Statistics | P-Value | Significance |
|---|---|---|---|
| Gender (male vs. female) | Z = -0.472 | 0.637 | Not significant |
| Marital status (single vs. married) | Z = -1.396 | 0.163 | Not significant |
| Insurance type | H = 2.230 | 0.526 | Not significant |
| Residence (urban vs. rural) | Z = -1.538 | 0.124 | Not significant |
| Employment status (employed vs. retired) | Z = -1.559 | 0.119 | Not significant (but component-level P-value) |
| SES | H = 3.872 | 0.144 | Not significant |
| Time since diagnosis | Z = -0.006 | 0.995 | Not significant |
| Presence of comorbidities | H = 0.004 | 0.948 | Not significant |
| Age group | HH = 8.828 | 0.184 | Not significant (but sleep efficiency P-value) |
Abbreviation: SES, socio-economic status.
5. Discussion
This cross-sectional study provides a detailed analysis of sleep quality and its multifaceted correlates in a specific cohort of OSCC patients in Ahvaz, Iran. The primary findings reveal an alarmingly high prevalence of poor sleep quality, significant pain levels, and a robust correlation between the two. Furthermore, our fine-grained analysis reveals that among a wide array of socio-demographic and clinical factors, employment status and age-specific variations in sleep efficiency emerged as significant, novel associates of specific sleep disturbance components, providing new insights for targeted interventions.
The mean global PSQI score of 9.64 in our cohort is notably high and aligns with a growing body of evidence documenting the severe sleep burden in head and neck cancer populations (15). The prevalence of poor sleep quality (84%) in our study exceeds that reported in some general cancer populations (18), underscoring the uniquely debilitating symptom profile of OSCC. Our component-level analysis adds critical nuance to this finding. The most severely affected domains were subjective sleep quality and sleep disturbances. This pattern suggests that the core issues for patients are not merely.
Quantitative sleep loss but a profound perception of their sleep as non-restorative, coupled with frequent nocturnal awakenings, characterizes the clinical picture. This is likely driven by a confluence of factors inherent to OSCC, including locoregional pain, xerostomia, nocturnal drooling, airway discomfort, and high levels of treatment-related anxiety. This aligns with the findings of Karimi et al. (3) in the same population, who reported significant deteriorations in oral health-related quality of life, domains of which are intrinsically linked to sleep comfort and function.
The strong positive correlation observed between pain intensity and global PSQI score (rs = 0.68) is a central finding of our study. This robust association firmly supports the well-established, bidirectional model of the pain-sleep relationship, wherein pain disrupts sleep architecture, and poor sleep, in turn, lowers pain thresholds and amplifies its perception through hyperalgesic mechanisms (12). In the specific context of OSCC, pain is often multifocal, involving somatic, visceral, and neuropathic components exacerbated by tumor invasion and treatment sequelae like mucositis and fibrosis (7, 8). Our results posit that unmanaged cancer-related pain acts as a primary driver of the sleep disruption epidemic in this population. This underscores the critical, yet potentially under-optimized, role of aggressive and proactive multi-modal pain management. A regimen that adequately addresses neuropathic components, beyond conventional analgesics, could be foundational to breaking this vicious cycle and improving overall sleep outcomes.
A novel and significant finding of this study is the pronounced impact of employment status on specific sleep dimensions. Employed patients reported significantly worse subjective sleep quality and greater daytime dysfunction compared to their unemployed counterparts. This can be interpreted through the lens of the "double burden" phenomenon, where patients are forced to manage the demands of a rigorous cancer treatment regimen alongside persistent job responsibilities, financial pressures, and the existential fear of job loss (30, 31). The chronic stress associated with maintaining work performance while contending with cancer-related fatigue, pain, and cognitive deficits likely exacerbates pre-sleep cognitive arousal (i.e., "racing thoughts"), impairing sleep initiation, and directly manifesting as excessive daytime sleepiness and dysfunction (32). This identifies employed OSCC patients as a particularly vulnerable subgroup that may benefit from targeted psychosocial and occupational interventions, such as structured workplace accommodations, flexible scheduling, and integration of fatigue management strategies into supportive care plans (33).
Furthermore, our analysis revealed that sleep efficiency varied significantly across age groups, being particularly poor in patients aged 55 - 64 years. This finding is intriguing as it deviates from a simple linear model of age-related sleep decline. This age range often coincides with peak career, financial, and familial responsibilities (the "sandwich generation"), potentially compounding the stress of a cancer diagnosis (34). It may also reflect a critical interaction where age-related changes in sleep architecture, such as reduced slow-wave sleep and increased sleep fragmentation, are potentiated by the physiological and psychological stress of OSCC and its treatment (35). This suggests a non-uniform impact of cancer on sleep across the adult lifespan and implies that sleep interventions may need tailoring; for instance, middle-aged patients might benefit most from Cognitive Behavioral Therapy for Insomnia (CBT-I) techniques focused on sleep consolidation and stimulus control (36).
Contrary to some literature linking broader SES to health outcomes (37, 38), we found no significant associations between global sleep quality and factors like insurance type, place of residence, or self-reported economic status in the multivariate model. This could indicate that within the context of our study, the universal and pervasive burden of the OSCC illness experience — its symptom severity and the standardized, often taxing, treatment protocols — overpowers the modulating influence of these broader socio-economic determinants. The profound nature of symptoms like pain and the psychological trauma of a head and neck cancer diagnosis may create a "ceiling effect" of distress, where sleep is uniformly and severely compromised across diverse social strata (39).
5.1. Clinical Implications and Future Research
The findings from this study carry several immediate clinical implications. First, routine and systematic screening for sleep disturbances using validated tools like the PSQI should be integrated into the standard of care for all OSCC patients. Second, management must be fundamentally multidisciplinary. Close collaboration between surgical, medical, and radiation oncologists with pain specialists, palliative care teams, and clinical psychologists is not optional but essential. Third, for employed patients, oncologists can play a vital role in advocating for necessary workplace supports and sick leave. Finally, evidence-based non-pharmacological interventions such as CBT-I, which has demonstrated efficacy in cancer populations (40), should be culturally adapted and made accessible within oncology care pathways in Iran.
5.2. Conclusions
In conclusion, this study reveals that poor sleep quality is a near-universal and severe problem among OSCC patients in Ahvaz, Iran, and is strongly intertwined with clinical pain. While pain is a central driver, the identification of employment status and specific age groups as modifiers of sleep disturbance introduces a layer of socio-demographic complexity that must be considered in clinical practice. Moving forward, a proactive, multi-dimensional, and tailored approach to supportive care is imperative to alleviate the interconnected suffering from sleep and pain, thereby improving the overall quality of life for patients battling these devastating diseases.
5.3. Limitations
This study has several limitations. The cross-sectional design precludes any causal inference about the relationships between pain, socio-demographic factors, and sleep quality. The use of a convenience sample from two tertiary centers may limit the generalizability of our findings to all OSCC patients in Iran, particularly those in rural areas or those not referred to specialized centers. While we used validated instruments, the data for sleep, pain, and psychological distress were self-reported and subject to recall and reporting biases. Furthermore, we did not objectively measure sleep with actigraphy or polysomnography, which could have provided more detailed data on sleep architecture. Despite these limitations, this study provides a crucial first comprehensive look into this complex interplay within a unique and understudied patient population. Additionally, the small sample size for subgroup analyses (e.g., age groups) limits the generalizability of those specific findings.