1. Background
Carob (Ceratonia siliqua), an evergreen tree in the Fabaceae family, is widely cultivated in the Mediterranean region due to its resistance to salt and drought. The tree is known for its sugar-rich pods and gum-containing seeds. Carob is also a source of minerals such as phosphorus, potassium, and iron, and is rich in vitamins E, D, and C, which contribute to human health (1, 2). Recent studies have focused on the effects of carob on human health, revealing significant quantities of various compounds with broad therapeutic applications (3). Polyphenolic compounds and antioxidant activity in carob have been shown to yield antifungal effects, reduce blood cholesterol, and provide anti-inflammatory and expectorant properties. Additionally, carob is effective in treating male infertility, cardiovascular diseases, and diarrhea (4). Some studies have utilized carob pods for bioethanol (5) or citric acid production (6). The polysaccharide-rich pods have high value in the food industry, while seeds containing tocopherols and plant sterols can counteract the damaging effects of free radicals, reducing oxidative stress (7, 8). Decoctions of carob leaves and bark have the highest total phenol content and can strongly inhibit acetylcholinesterase and butyrylcholinesterase enzymes (9). Carob pod essential oil displays moderate to strong antimicrobial activity against certain bacteria, such as Listeria monocytogenes, and fungi (10). Studies on the antibacterial properties of various medicinal plants have demonstrated that plant extracts are generally more effective against gram-positive bacteria than gram-negative bacteria, with Bacillus subtilis being the most sensitive and Escherichia coli the most resistant (11). A major health challenge is the use of chemical drugs and antibiotics to treat bacterial infections, which can lead to side effects and drug resistance. The use of native species is therefore a key research goal in developing more effective drugs with fewer side effects (11). Carob seeds have shown antiproliferative effects and have inhibited the growth of various cancer cell lines, including MCF-7 and HeLa (12). The anticancer mechanisms may involve mitochondrial or intrinsic apoptosis, regulated by members of the Bcl-2 superfamily, which block mitochondrial membrane pore permeability. Following apoptosis induction, Bcl-2 levels decrease, pro-apoptotic factors are released from the mitochondria, and caspase-9 is activated, leading to the activation of downstream caspases 3, 6, and 7 (13). Many natural agents can also induce caspase-independent apoptosis (CI-PCD) through cleavage of the apoptosis-inducing factor (AIF) (14).
2. Objectives
Given the therapeutic and nutritional value of carob for human health, this study aimed to evaluate the potential therapeutic properties of carob seed extract, focusing on its antioxidant, antibacterial, and inhibitory effects on the growth of MCF-7 cancer cells.
3. Methods
3.1. Sample Preparation and Extraction
Carob seeds were purchased in Kermanshah, Iran, in May. After identification by the Department of Biology at Lorestan University, the seeds were separated from the dried pods and ground. Five grams of the powdered seeds were weighed and heated with 10 mL of distilled water for 10 minutes. The resulting solution was filtered and centrifuged at 9000 rpm for 15 minutes. The extract was then filtered again, yielding the aqueous extract.
3.2. Fourier Transform Infrared Spectroscopy
Fourier transform infrared spectroscopy (FTIR) was used to identify the organic compounds and functional groups in the extract. Dried carob seed powder was mixed with potassium bromide (KBr) in a 1:100 ratio, pressed into a transparent pellet using a hydraulic press, and analyzed using an FTIR spectrophotometer (wavelength range: 400 to 4000 cm-1).
3.3. Determination of Total Phenolic Content
The total phenolic content of the aqueous extract was determined using the Folin-Ciocalteu method. The extract was mixed with Folin-Ciocalteu reagent and sodium carbonate solution, incubated at 30°C for 90 minutes, and the absorbance was measured at 765 nm. Results are expressed as mg gallic acid equivalents per gram of dry extract (15).
3.4. Determination of Total Flavonoid Content
The total flavonoid content was determined using aluminum chloride colorimetry. The extract was mixed with sodium nitrite, aluminum chloride, and sodium hydroxide, and the absorbance was measured at 510 nm. Results are expressed as mg quercetin equivalents per gram of dry extract (16).
3.5. Antibacterial Activity by Disk Diffusion Method
The antibacterial activity of the extract was tested against gram-negative bacteria, including E. coli (ATCC25922), Staphylococcus aureus (ATCC2911), Klebsiella (ATCC3521), and Pseudomonas aeruginosa (ATCC1181). Bacteria were cultured on Mueller-Hinton agar, and the inhibition zone diameter was measured after 24 hours of incubation at 37°C (16, 17).
3.6. Minimum Inhibitory Concentration
The minimum inhibitory concentration (MIC) was determined using the microdilution broth method in 96-well microplates. The extract was serially diluted, and bacterial suspension was added to each well. After 24 hours of incubation at 37°C, the MIC was defined as the lowest extract concentration that inhibited bacterial growth (18).
3.7. Minimum Bactericidal Concentration
The minimum bactericidal concentration (MBC) was determined as the lowest extract concentration that killed 99.9% of the bacteria. Bacteria from wells showing no turbidity were cultured on Mueller-Hinton agar and incubated at 37°C for 24 hours (18).
3.8. MTT Assay
The MCF-7 breast cancer cell line was obtained from the Pasteur Institute of Iran and cultured in a T25 flask. Cells were maintained in a CO2 incubator at 37°C and 95% humidity. The cytotoxic effect of the carob seed extract was evaluated using the MTT assay. Cells were treated with different extract concentrations (50, 100, 150, and 200 μg/mL) for 24, 48, and 72 hours. After incubation, MTT solution was added, and absorbance was measured at 570 nm using an ELISA reader. Data were analyzed using Excel 2016 software, ANOVA, and Duncan's post-hoc test.
4. Results
4.1. Fourier Transform Infrared Spectroscopy Analysis
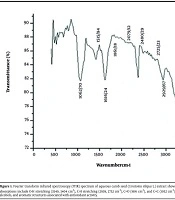
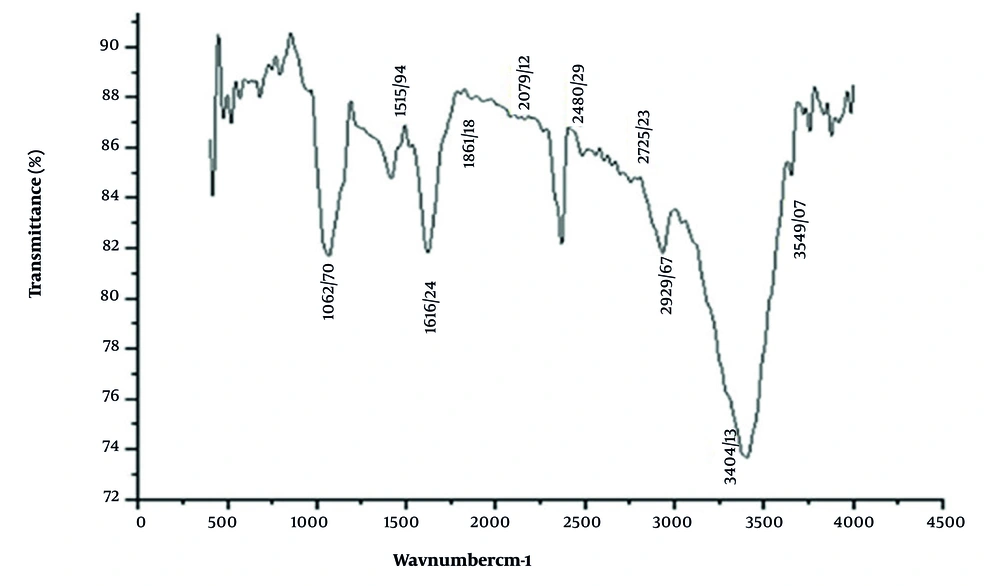
The FTIR spectrum revealed key peaks at 3549, 3404, 2939, 2752, 1616, and 1052 cm-1, corresponding to functional groups such as O-H (alcohols, phenols, or N-H in amines), C-H (alkanes and aldehydes), C=O (carboxyl or amide groups), and C=C (ketones and aromatic compounds). These molecular vibrations suggest the presence of biomolecules potentially linked to the plant’s antioxidant properties (Figure 1).
Fourier transform infrared spectroscopy (FTIR) spectrum of aqueous carob seed (Ceratonia siliqua L.) extract showing characteristic functional group peaks; notable absorptions include O-H stretching (3549, 3404 cm-1), C-H stretching (2939, 2752 cm-1), C=O (1616 cm-1), and C=C (1052 cm-1), indicating the presence of phenolic compounds, alcohols, and aromatic structures associated with antioxidant activity.
4.2. Phenolic and Flavonoid Content
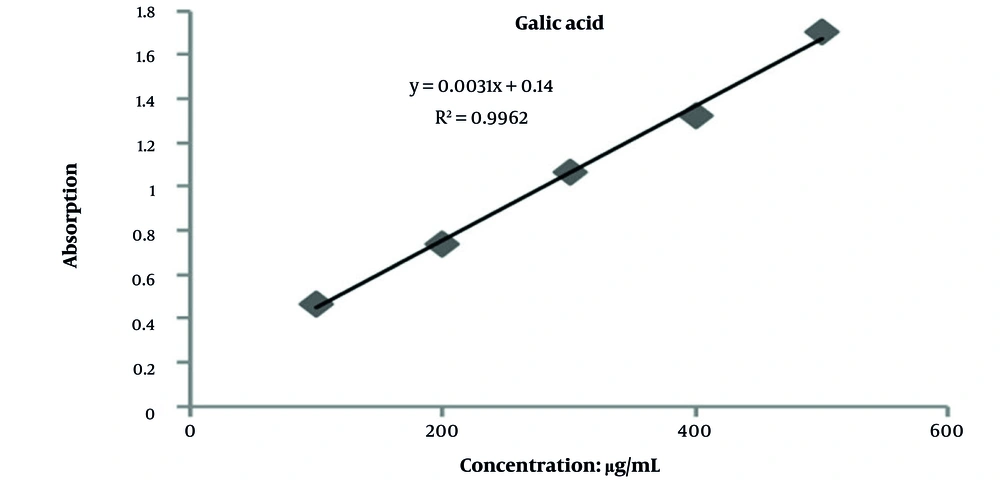
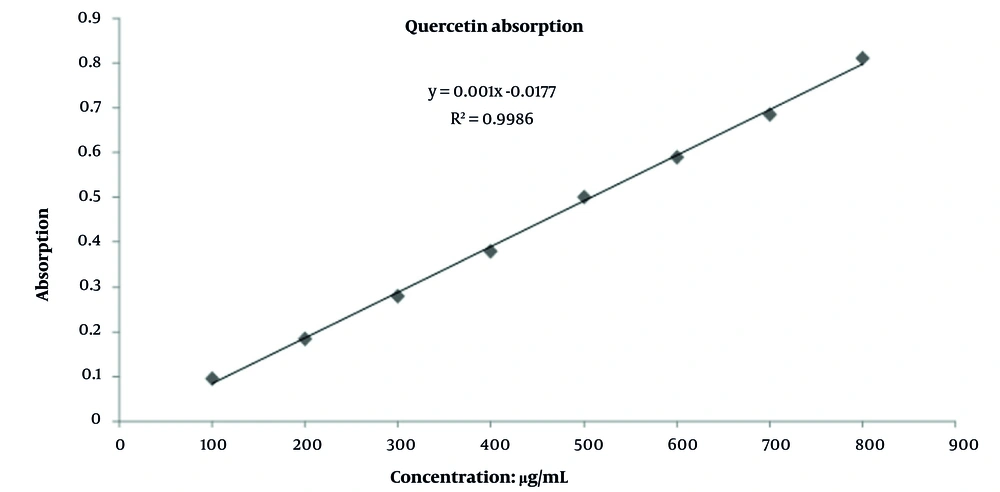
The total phenolic content in the aqueous extract of carob seeds, determined using the Folin-Ciocalteu method, was 184.83 ± 0.5 mg gallic acid equivalents per gram of dry extract. The total flavonoid content, measured via the aluminum chloride colorimetric method, was 104.61 ± 0.3 mg quercetin equivalents per gram of dry sample (Figures 2 and 3).
Standard calibration curve of gallic acid for quantification of total phenolic content in carob seed extract; absorbance was measured at 765 nm after the Folin-Ciocalteu assay. The linear regression (R2 > 0.99) was used to calculate phenolic content as 184.83 ± 0.5 mg gallic acid equivalents per gram of dry extract.
Standard calibration curve of quercetin for determination of total flavonoid content in carob seed extract; absorbance was measured at 510 nm using aluminum chloride colorimetry. The linear regression (R2 > 0.99) yielded flavonoid content of 104.61 ± 0.3 mg quercetin equivalents per gram of dry extract.
4.3. Antibacterial Activity
The aqueous extract demonstrated concentration-dependent antibacterial activity, with a more pronounced effect against gram-negative bacteria. The highest inhibition zone diameter (14.22 ± 0.22 mm) was observed against P. aeruginosa. The MIC and MBC values for P. aeruginosa were 32.5 μg/mL and 125 μg/mL, respectively, indicating stronger inhibitory effects against gram-negative bacteria compared to gram-positive strains (Tables 1 and 2).
| Bacteria/Concentration | 100 | 500 | 100 | Amikacin/Control |
|---|---|---|---|---|
| Escherichia coli | 5 ± 0.4 | 8 ± 0.8 | 11.22 ± 0.4 | 13.11 |
| Staphylococcus aureus | 6 ± 0.4 | 9.4 ± 0.9 | 12.33 ± 0.12 | 14.44 |
| Klebsiella | 0 ± 0.1 | 5 ± 0.1 | 8.5 ± 0.5 | 11.22 |
| Pseudomonas aeruginosa | 9.8 ± 0.8 | 12 ± 0.1 | 14.22 ± 0.22 | 15.66 |
a Values are expressed as mean ± SD.
| Bacterias | MBC | MIC |
|---|---|---|
| Escherichia coli | 125 | 125 |
| Staphylococcus aureus | 250 | 125 |
| Klebsiella | 250 | 125 |
| Pseudomonas aeruginosa | 125 | 32.5 |
Abbreviations: MBC, minimum bactericidal concentration; MIC, minimum inhibitory concentration.
4.4. Cytotoxic Effect on MCF-7 Cell Line
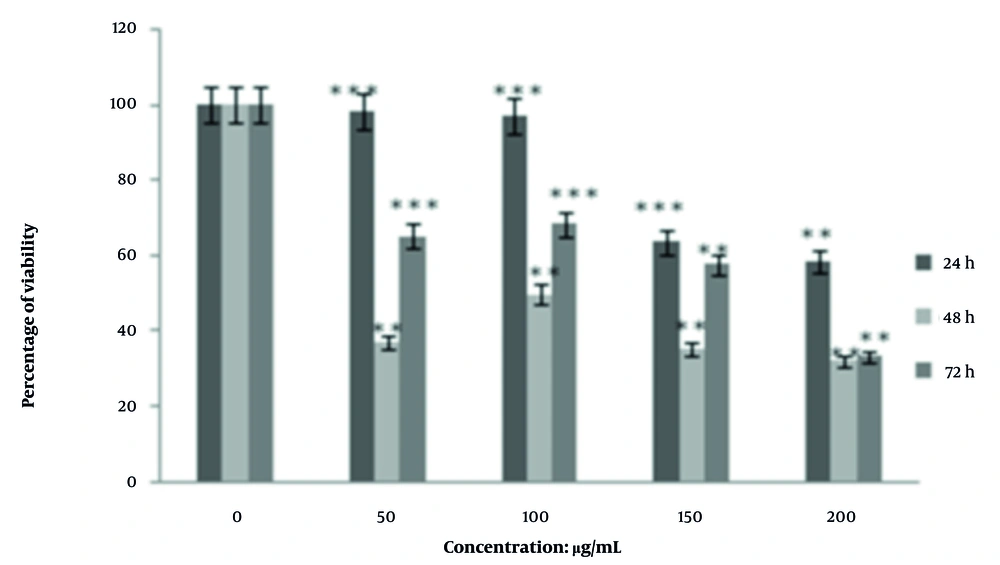
The IC50 value at 48 hours was lowest at 100 μg/mL, indicating maximal inhibitory and cytotoxic effects at this concentration. However, at 72 hours, the inhibitory effect decreased with increasing concentration, with no linear concentration-activity relationship observed. The most significant inhibitory effect across time points was seen at 50 μg/mL (P < 0.05), suggesting that lower concentrations may be more effective over extended periods (Figure 4).
Inhibitory effect of aqueous carob seed extract on the viability percentage of the MCF-7 cell line; results are based on mean ± standard deviation of data at concentrations (50, 100, 150, and 200 μg/mL) and at three time points (24, 48, and 72 hours; ** significant difference at P < 0.05 compared to the control group at different time points; *** significant difference at P < 0.05 at different concentrations compared to the control group).
5. Discussion
Natural plants have evolved with optimal chemical compounds effective against a wide range of diseases, including cancer and diabetes. Recent studies have highlighted that carob contains significant amounts of polyphenols, flavonoids, gallic acid, and myricetin, all of which have detoxifying, antioxidant, and anticancer properties (3). The FTIR analysis in this study confirmed the presence of these functional groups in carob seeds, which may contribute to their antiproliferative and antioxidant properties. Other studies have shown that the composition of these compounds varies with the plant's growth stage, with immature carob containing high levels of gallotannins and catechins, conferring anti-inflammatory effects and protection against oxidative stress-related diseases (19). Mokhtari et al. demonstrated that reducing free radicals through the consumption of antioxidant-rich substances can minimize oxidative stress and reduce the complications of diabetes (20).
This study also showed that carob seed extract has strong antibacterial and antimicrobial properties, particularly against gram-negative bacteria such as P. aeruginosa. The antibacterial activity of the extract was concentration-dependent, suggesting that plant extracts could serve as suitable alternatives to chemical antibiotics and have fewer side effects. Hsouna et al. reported that carob pod essential oil possesses antimicrobial and cytotoxic properties, consistent with the findings of this study (10). Meziani et al. found that acetone and ethanol extracts of carob leaves and pods have significant inhibitory effects against gram-negative bacteria, in agreement with our results (21). Aissani et al. demonstrated that methanolic extracts of carob leaves inhibit the growth of L. monocytogenes (22).
This study also confirmed the antiproliferative effects of carob seeds on the MCF-7 cancer cell line, with a 50% reduction in cell viability, supporting previous findings and indicating the presence of anticancer compounds. However, the relationship between concentration and antiproliferative activity was not linear, with lower concentrations showing greater inhibitory effects. This non-linear behavior may be attributed to hormesis, where low concentrations of natural compounds such as polyphenols or flavonoids produce more beneficial effects (23). Further studies have shown that carob seeds exert antiproliferative effects on breast cancer cells without affecting normal cells, with the inhibitory mechanism involving both caspase-dependent and -independent apoptosis. Additionally, carob seeds, with their high antioxidant potential, can neutralize free radicals, preventing damage to cells and DNA (12). Raeisalsadati et al. demonstrated that zinc oxide nanoparticles synthesized with carob extract have cytotoxic effects on breast cancer cells and inhibit angiogenesis (24). Ben Ayache et al. evaluated the biological activity of aqueous extracts from carob syrup, demonstrating pro-apoptotic effects on human cancer cell lines, including MCF-7 (25). While many laboratory studies have shown that certain compounds in carob may inhibit the growth of cancer cells, including breast cancer, these results are primarily from preclinical studies, and further human research is necessary to confirm their efficacy and determine safe and effective doses. In the meantime, carob can be considered a healthy and beneficial dietary component.
5.1. Conclusions
This study demonstrated that carob seed extract, owing to its high phenolic, flavonoid, and antioxidant content, can be used as an antibacterial agent (particularly against gram-negative bacteria) and as an anticancer agent against the MCF-7 cell line, although further research is warranted.