1. Background
Oxidative stress is known to cause damage to biological molecules such as proteins, lipids, amino acids, and nucleic acids (1). Studies have shown that free radicals slow down the function of neurons and cause a variety of neurological disorders (2, 3). Several natural substances have demonstrated efficacy against neurodegeneration (4). Herbal extracts may be preferred over purified phytoconstituents due to their potential ability to exert diverse biological effects and lower toxicity (5). Artemisia turanica Krasch. is one of the naturally growing plant species in Iran, belonging to the family Asteraceae (6). The ethyl acetate (EA) extract of A. turanica has been effective in in vitro antioxidant assays (7).
2. Objectives
This study was designed to compare the cytoprotective potential of different fractions of the EA extract of A. turanica Krasch. against oxidative stress and apoptosis in PC12 cells, which is an accepted model for in vitro neuroprotective studies. The extract was previously reported to be active in cytoprotective assays.
3. Methods
3.1. Materials and Reagents
2′,7′-Dichlorodihydrofluorescein diacetate (DCF), 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Triton-X-100, dimethyl sulfoxide (DMSO), and Rhodamine 123 were purchased from Sigma Aldrich (St. Louis, MO). Additionally, Trypsin-EDTA was supplied by Bon Yakhteh, Iran. Fetal bovine serum was purchased from Gabon, USA. RP18 (15 - 25 μm) and methanol were purchased from Merck, Germany. PC12 pheochromocytoma cells were provided by the Pasteur Institute of Iran (Tehran, Iran).
3.2. Preparation of Plant Extract and Fractions
The aerial parts of A. turanica were collected from Samie Abad, Torbat-e Jam, Razavi Khorasan province, Iran. A voucher specimen (with the identification number 12572) has been deposited in the Herbarium of Mashhad University of Medical Sciences. A total of 160 g of dried and ground plant material was extracted with petroleum ether (40 - 60), dichloromethane, and EA using the maceration method. The EA extract was concentrated under reduced pressure at a maximum temperature of 45°C. The dried extract was fractionated by the reversed-phase VLC method with different ratios of methanol (20 - 100%) in water as a mobile phase to afford six fractions (F1 - F6). The procedure was followed by using pure acetone as the eluting solvent to obtain F7.
3.3. Determination of Total Phenolic and Flavonoid Content of Fractions
The Folin-Ciocalteu method was used to determine the total phenolic content (TPC) of fractions (8). The TPC was expressed as mg of gallic acid equivalent (GAE) per gram of dried weight of the fractions. The calculation of the total flavonoid content (TFC) of fractions was performed using the colorimetric method with aluminum chloride (9). The TFC was expressed as mg of quercetin equivalent (QE) per gram of dried weight of the fractions.
3.4. Determination of Cytotoxicity Effects of Different Fractions of the Ethyl Acetate Extract and Hydrogen Peroxide by MTT Assay
The MTT method (10) was used to determine the cytotoxicity of hydrogen peroxide (H2O2) and plant fractions against PC12 cells. The IC50 value was described as the concentration at which 50% of the cells were killed.
3.5. Determination of the Protective Effect of Fractions Against Hydrogen Peroxide-Induced Cytotoxicity
PC12 cells were pretreated with non-toxic concentrations of different plant fractions (determined after the MTT study) for 24 hours and subsequently treated with H2O2 (5.0 mg/mL) for another 24 hours. The MTT method (10) was used to determine the protective effect of fractions against H2O2-induced cytotoxicity.
3.6. Evaluation of Intracellular Reactive Oxygen Species
The intracellular reactive oxygen species (ROS) was evaluated using the DCF-DA indicator (10). The intracellular ROS formation was studied in three groups of cells: (A) control, (B) cells treated with H2O2 (5.0 mg/mL) for 24 hours, and (C) cells pretreated with a non-toxic concentration of the selected fraction (2.5 μg/mL) for 24 hours and subsequently treated with H2O2 (5.0 mg/mL) for another 24 hours.
3.7. Measurement of Mitochondrial Membrane Potential
The cells were seeded in 6-well tissue culture plates and incubated for 24 hours, after which the selected fraction (2.5 μg/mL) was added to the wells. After 24 hours, the IC50 of H2O2 was added to the cells and incubated for another 4 hours. At the end of the treatment, the mitochondrial membrane potential (MMP) was assessed using Rhodamine 123 as a fluorescent dye (7).
3.8. Caspase-3 Activity Assay
The PC12 cells were seeded in 6-well tissue culture plates and incubated for 24 hours. Then, the selected fraction (2.5 μg/mL) was added to the wells and incubated for the next 24 hours. The H2O2 (5.0 mg/mL) was added to the treated cells and incubated for a further 4 hours. Caspase-3 activity was determined using the Sigma colorimetric caspase kit (7).
4. Results
4.1. Total Phenolic and Flavonoid Contents of Fractions
The TPC (mg of GAE/g of each fraction) and TFC (mg of QE/g of each fraction) values are presented in Table 1. The TFC for the EA fractions varied from 13.89 ± 0.42 (F7) to 236.11 ± 4.86 (F2), while TPC values varied from 14.25 ± 0.25 (F7) to 352.69 ± 27.08 (F2).
| Sample | TFC (mg of QE/g) | TPC (mg of GAE/g) |
|---|---|---|
| F1 | 23.73 ± 0.81 | 146.89 ± 1.26 |
| F2 | 236.11 ± 4.86 | 352.69 ± 27.08 |
| F3 | 139.87 ± 3.25 | 144.67 ± 22.40 |
| F4 | 112.99 ± 1.23 | 115.34 ± 8.47 |
| F5 | 58.97 ± 1.11 | 62.45 ± 1.92 |
| F6 | 31.8 ± 0.42 | 33.41 ± 0.36 |
| F7 | 13.89 ± 0.42 | 14.25 ± 0.25 |
Abbreviations: TFC, total flavonoid content; QE, quercetin equivalent; TPC, total phenolic content; GAE, gallic acid equivalent.
a Values are expressed as mean ± SD.
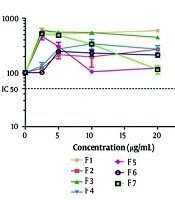
4.2. Cytotoxicity of Hydrogen Peroxide and Fractions on PC12 Cells
MTT results showed that the IC50 value of H2O2 was 5.0 mg/mL. All the fractions of the EA extract (at concentrations up to 20 μg/mL) did not induce any toxicity in PC12 cells (Figure 1).
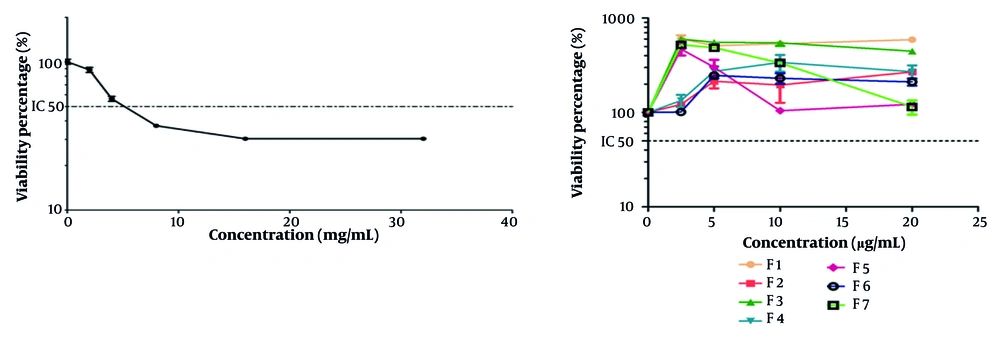
4.3. The Protective Effect of Fractions on Hydrogen Peroxide-Induced Cytotoxicity
F6, at a concentration of 2.5 μg/mL, had a significant protective effect against the oxidative stress induced by H2O2 (Figure 2).
4.4. The Effect of Selected Fraction on Hydrogen Peroxide-Induced Intracellular Reactive Oxygen Species Generation
According to Figure 3, ROS generation increased significantly after treatment of cells with the IC50 of H2O2. F6, at a concentration of 2.5 μg/mL, had a significant inhibitory effect on ROS production.
Effect of pre-exposure to the selected fraction of the ethyl acetate (EA) extract of Artemisia turanica on the induction of reactive oxygen species (ROS) in PC12 cells [data are presented as the mean ± SEM, n = 3; ### P < 0.001 significant differences compared to the hydrogen peroxide (H2O2)-treated group].
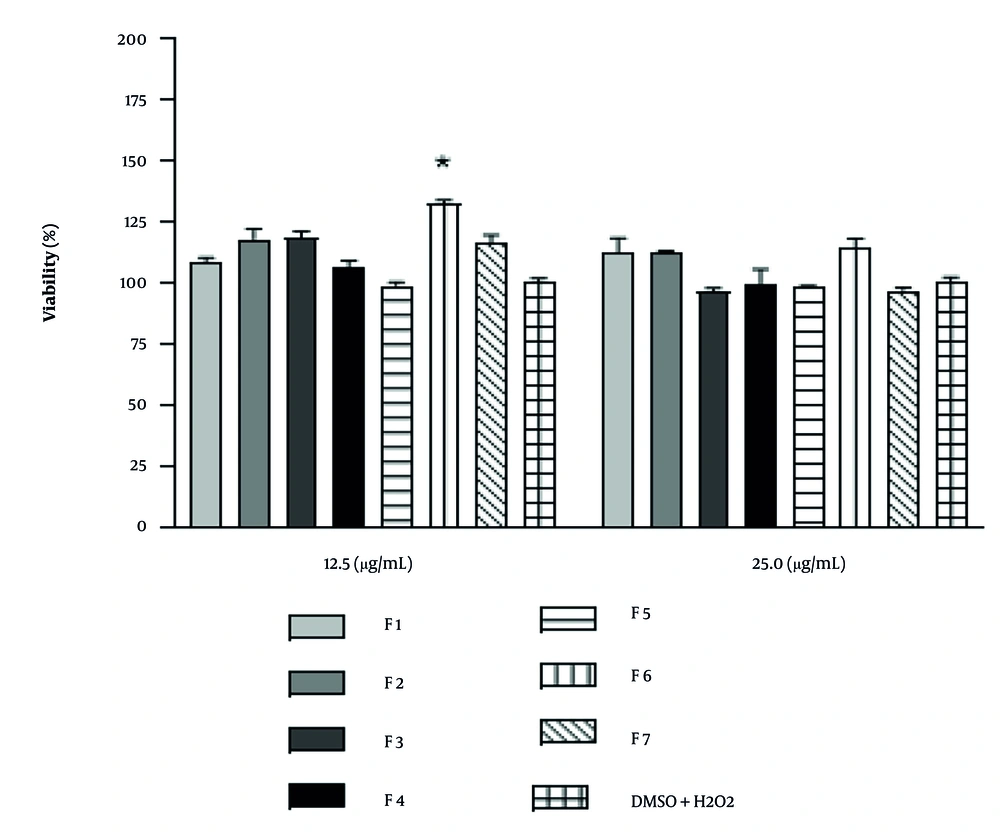
4.5. Effect of Selected Fraction on the Mitochondrial Membrane Potential
As seen in Figure 4, F6 (2.5 μg/mL) was not able to significantly inhibit the reduction of MMP.
4.6. Effect of Selected Fraction on Caspase-3 Activity
According to Figure 5, pretreatment of cells with F6 at a concentration of 2.5 μg/mL significantly decreased the activity of caspase-3.
5. Discussion
In the present study, the cytoprotective potential of different fractions of the EA extract of A. turanica on H2O2-induced oxidative stress and apoptosis in PC12 cells was investigated. This extract was selected based on the results of our previous study (7). Similar studies have reported the neuroprotective potential of other Artemisia species, such as A. princeps and A. absinthium L., via antiapoptotic activity and inhibition of intracellular ROS production (11, 12). The antioxidant capacity of plants is mostly attributed to their polyphenolic components (13).
Sterols, polyacetylenes, and terpenoids are some of the major non-phenolic constituents in the genus Artemisia (14). In the current study, the TPC and TFC of F6 — the selected fraction, based on in vitro neuroprotectivity — were relatively low. This finding indicates that non-phenolic components of this fraction may play a role in the bioactivity. Some non-phenolic secondary metabolites, such as terpenoids (15), polyacetylenes (16), and alkaloids (17) ,have shown notable antioxidant and neuroprotective effects. However, a phytochemical investigation can identify the key compounds of this fraction.
5.1. Conclusions
The neuroprotective fraction of the EA extract of A. turanica is probably rich in non-phenolic antioxidant components.
![Effect of pre-exposure to the selected fraction of the ethyl acetate (EA) extract of <i>Artemisia turanica</i> on the induction of reactive oxygen species (ROS) in PC12 cells [data are presented as the mean ± SEM, n = 3; ### P < 0.001 significant differences compared to the hydrogen peroxide (H<sub>2</sub>O<sub>2</sub>)-treated group]. Effect of pre-exposure to the selected fraction of the ethyl acetate (EA) extract of <i>Artemisia turanica</i> on the induction of reactive oxygen species (ROS) in PC12 cells [data are presented as the mean ± SEM, n = 3; ### P < 0.001 significant differences compared to the hydrogen peroxide (H<sub>2</sub>O<sub>2</sub>)-treated group].](https://services.brieflands.com/cdn/serve/3170f/8962f3d029ff35414f52c22f48b90a304068c9de/jjcmb-16-4-161752-i003-preview.webp)
![Effect of pre-exposure to the selected fraction of the ethyl acetate (EA) extract of <i>Artemisia turanica</i> on the reduction of caspase-3 activity [data are presented as the mean ± SEM, n = 3; ## P < 0.01 significant differences compared to the hydrogen peroxide (H<sub>2</sub>O<sub>2</sub>)-treated group]. Effect of pre-exposure to the selected fraction of the ethyl acetate (EA) extract of <i>Artemisia turanica</i> on the reduction of caspase-3 activity [data are presented as the mean ± SEM, n = 3; ## P < 0.01 significant differences compared to the hydrogen peroxide (H<sub>2</sub>O<sub>2</sub>)-treated group].](https://services.brieflands.com/cdn/serve/3170f/4ae359c0adf9f96c12ff00674b58a71b683a8e33/jjcmb-16-4-161752-i005-preview.webp)