1. Context
1.1. What Is a Uterine Fibroid?
Uterine fibroids are caused by changes in the smooth muscle cells of the uterine wall and typically develop before menopause, during the reproductive years. The prevalence of fibroids is between 70% to 80% (1), and they affect over 25% of women. Fibroids are classified based on their size and location. They usually do not present with specific symptoms, but in some women, they are associated with heavy bleeding, fertility issues (whether naturally or through IVF), pelvic pain, and more (2). It is important to note that heavy bleeding can lead to various medical problems, including iron deficiency and anemia. The occurrence of most complications is directly related to the location and size of the fibroid. For example, large fibroids — referred to as "bulk" — can increase the size of the uterus and disrupt bladder function (3). One method of diagnosing uterine fibroids is by feeling for abnormal bulges and indentations in the pelvic area. To confirm the diagnosis and differentiate it from pregnancy, ultrasound imaging is used (4). In this article, we examine the cellular and molecular mechanisms involved in the formation of uterine fibroids.
2. Evidence Acquisition
In this review study, to find relevant articles about the cellular and molecular mechanisms involved in the development of uterine fibroids, published studies from 1995 onwards — including clinical trials, observational, and descriptive studies — were searched in the databases Google Scholar, Scopus, PubMed, SID, and Magiran using the keywords: Uterine fibroid, cellular and molecular mechanisms, and genetic mutations.
3. Results
3.1. Causes of Uterine Fibroids
The causes of uterine fibroids include obesity, age, race, smoking, poor diet, cosmetic and hygiene products, blood pressure, family history, and genetic mutations. The prevalence of uterine fibroids in Black women is about 10% higher than in White women. This is because Black women are more likely to be exposed to parabens and phthalates through cosmetic and hygiene products (5). Additionally, the incidence of this disease is higher in individuals who consume large amounts of red meat in their diet. People with a history of high blood pressure are also more likely to be affected by this condition. Recent studies have pointed to a direct relationship between the use of cosmetic and hygiene products and an increased likelihood of fibroid development (3, 6-10).
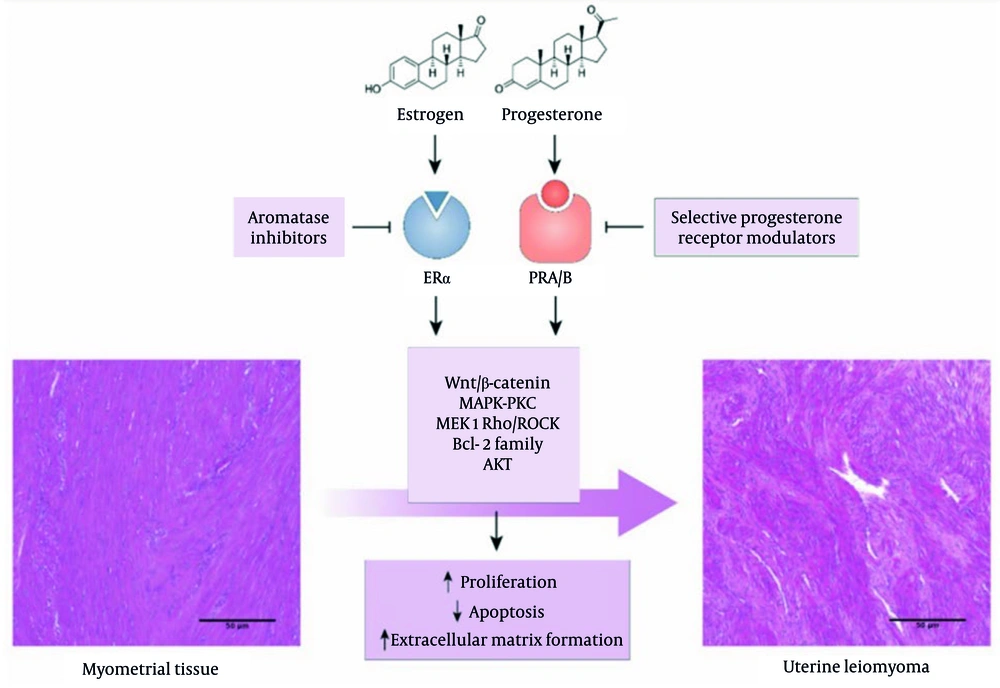
Additionally, disruptions in estrogen and progesterone hormone levels play an important role in the growth of fibroids. These hormones can stimulate cell division in the uterine muscle tissue, leading to fibroid growth (Figure 1).
Investigation of the effects of estrogen and progesterone on myometrial tissue (11)
Recent research has examined the role of the endometrial and vaginal microbiome in uterine diseases such as fibroids. An imbalance in the microbiome may lead to improper immune regulation and inflammation, creating conditions favorable for fibroid growth. Various cellular and molecular factors also play a role in the formation of these fibroids. Uterine fibroids can even alter gene expression patterns (for example, HOX10) and disrupt implantation and fertility (12).
3.2. Cellular and Molecular Mechanisms Underlying Uterine Fibroids
The cellular and molecular mechanisms in uterine fibroids are complex and involve hormonal interactions, genetic alterations, and various signaling pathways. Understanding these mechanisms can lead to advances in effective treatments for these common tumors. Genomic research has shown that certain genes are involved in the development of myomas. For example, alterations in the genes MED12, HMGA2, TP53, and COL1A1 have been identified as evidence of genetic diversity in myomas. Studies have shown that genetic mutations in these genes can disrupt cellular signaling and cause changes in the cell cycle.
Generally, mutations in the MED12 gene are observed in many fibroids and can lead to disturbances in cellular signaling. HMGA2 is involved in regulating the expression of genes related to cell growth and differentiation. COL1A1 plays a role in collagen production and can affect the connective tissue in fibroids, while TP53 participates in cell cycle control and DNA repair. Genetic alterations in these genes can influence fibroid growth through various signaling pathways, including MAPK, PI3K/AKT, and Wnt/β-catenin, by stimulating cell division or preventing cell death.
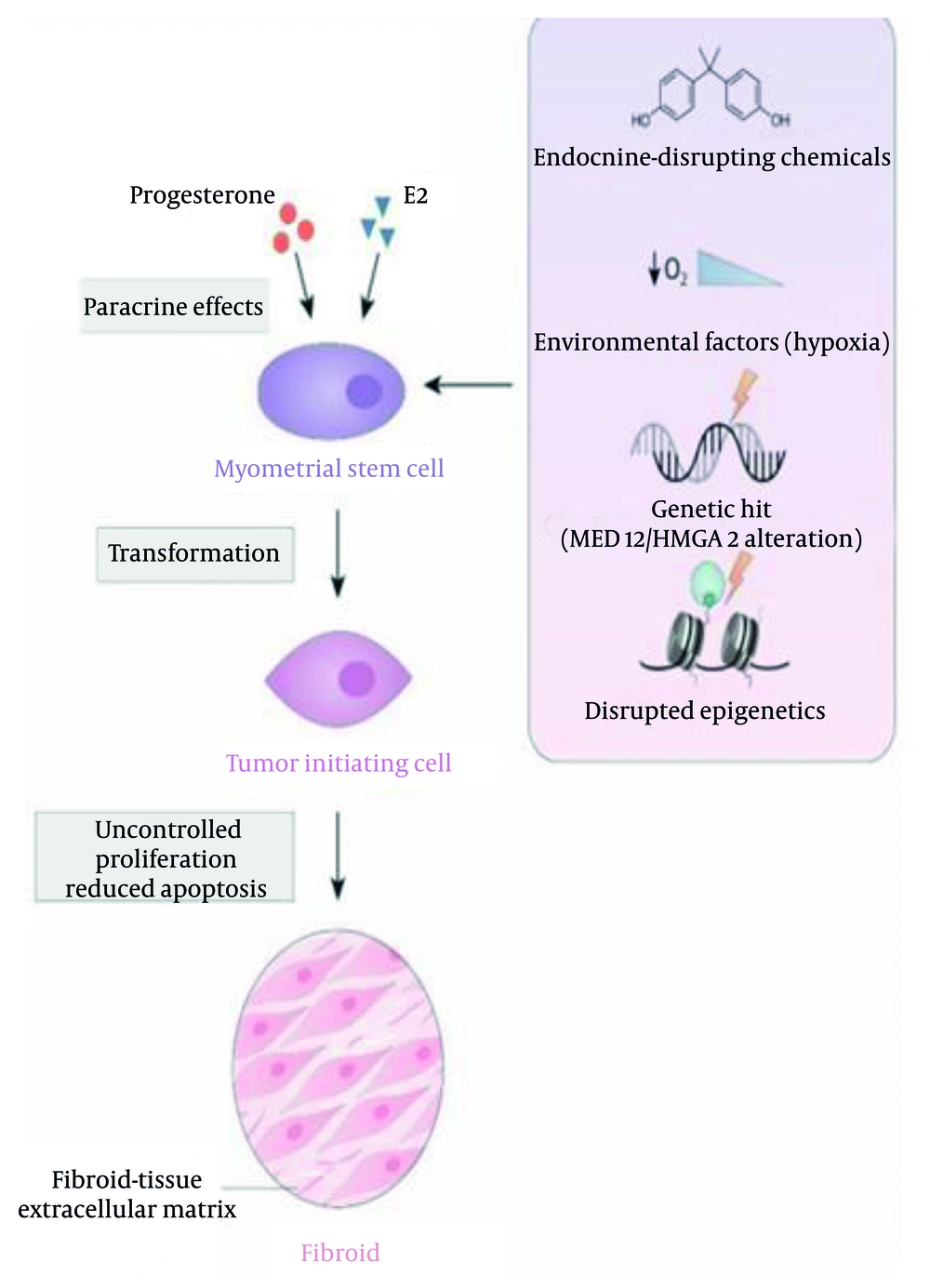
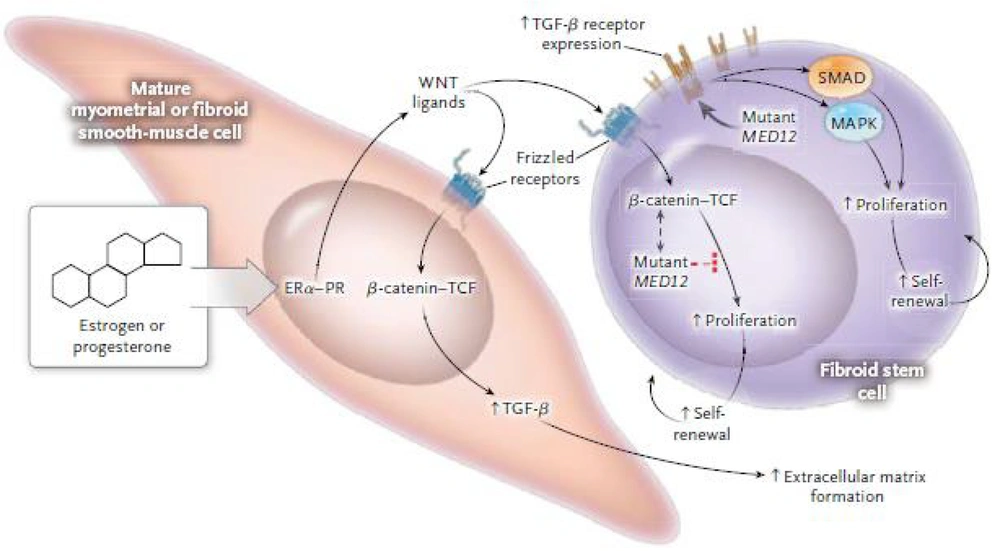
For instance, fibroid stem cells harbor point mutations in MED12 or increased expression of HMGA2, indicating a genetic hit that transforms the initial stem cell (Figure 2). This hit can cause changes and interference in various pathways such as cellular signaling, cell proliferation, survival, senescence, and more. Cells lacking MED12 mutations or increased HMGA2 expression are called non-MED12-HMGA2 (13). How exactly the mutation and alteration in MED12 lead to fibroid formation is still unknown (14). Increased β-catenin or other disruptions in transforming growth factor beta (TGF-β) expression occur as a result of one of these hits (Figure 3) (15). High levels and activation of β-catenin in uterine mesenchyme during both embryonic development and in adult mice cause the formation of fibroid-like tumors.
Cellular origin of uterine fibroid cells (11)
Interactions between ovarian hormones, β-catenin, and transforming growth factor beta (TGF-β) pathways, and MED12 in fibroid cells (15)
The group of HMGI proteins encoded by the HMGA1 and HMGA2 genes are primarily expressed during fetal development in various tissues and become inactive after maturation. HMGA proteins can bind to specific regions of DNA called AT hooks. The formation of these hooks in chromatin causes structural changes and simultaneously allows the regulation of expression of various target genes (16). HMGA2 is highly expressed in many types of neoplasms, especially in uterine fibroids. Previous studies have established a correlation between overexpression of HMGA2 and larger tumor size (17). It should also be noted that no clear association has been found between the levels of EGR-1 and HMGA2 in fibroids (18). The let-7 family, which is part of microRNAs, increases to suppress HMGA2 expression in cells (19). However, as fibroid size increases, HMGA2 expression markedly rises while let-7 levels decrease. Therefore, the lack of pairing between let-7 and HMGA2 is one of the key molecular mechanisms promoting tumor growth (17). RAD51L1 is one of HMGA2’s powerful partners in fibroid development. RAD51L1 is involved in DNA recombination. Other partners of HMGA2 include the genes COX6X, HEI10, ALDH2, and RTVK-H3 (20). The PCOLCE gene is a region disrupted in leiomyomata disease and plays a role in controlling cell growth and differentiation (21).
The TGF-β factors are multifunctional growth factors. They regulate many cellular functions such as proliferation, differentiation, extracellular matrix (ECM) production, and more (22). In humans, three types of active TGF-β exist, and the expression of all three types along with their receptors has been identified in human myometrium tissue as of 2024/10/25. Scientists, through various studies, have observed that the expression of TGF-β and its receptors, including their intracellular signaling pathways, is significantly higher in fibroids compared to the myometrium (18). The TGF-β can contribute to fibroid growth by increasing cell proliferation and ECM production (22).
Recent studies indicate that uterine fibroids may be associated with a chronic inflammatory condition in the myometrial tissue. Elevated levels of interleukin-6 (IL-6), TNF-α, and activated macrophages have been observed in fibroid tissues. These inflammatory mediators can regulate the expression of genes related to fibroid growth. There is evidence that lncRNA H19, MALAT1, and some circRNAs play roles in regulating cell proliferation and migration of fibroid cells. These molecules have been proposed as potential biomarkers for early detection.
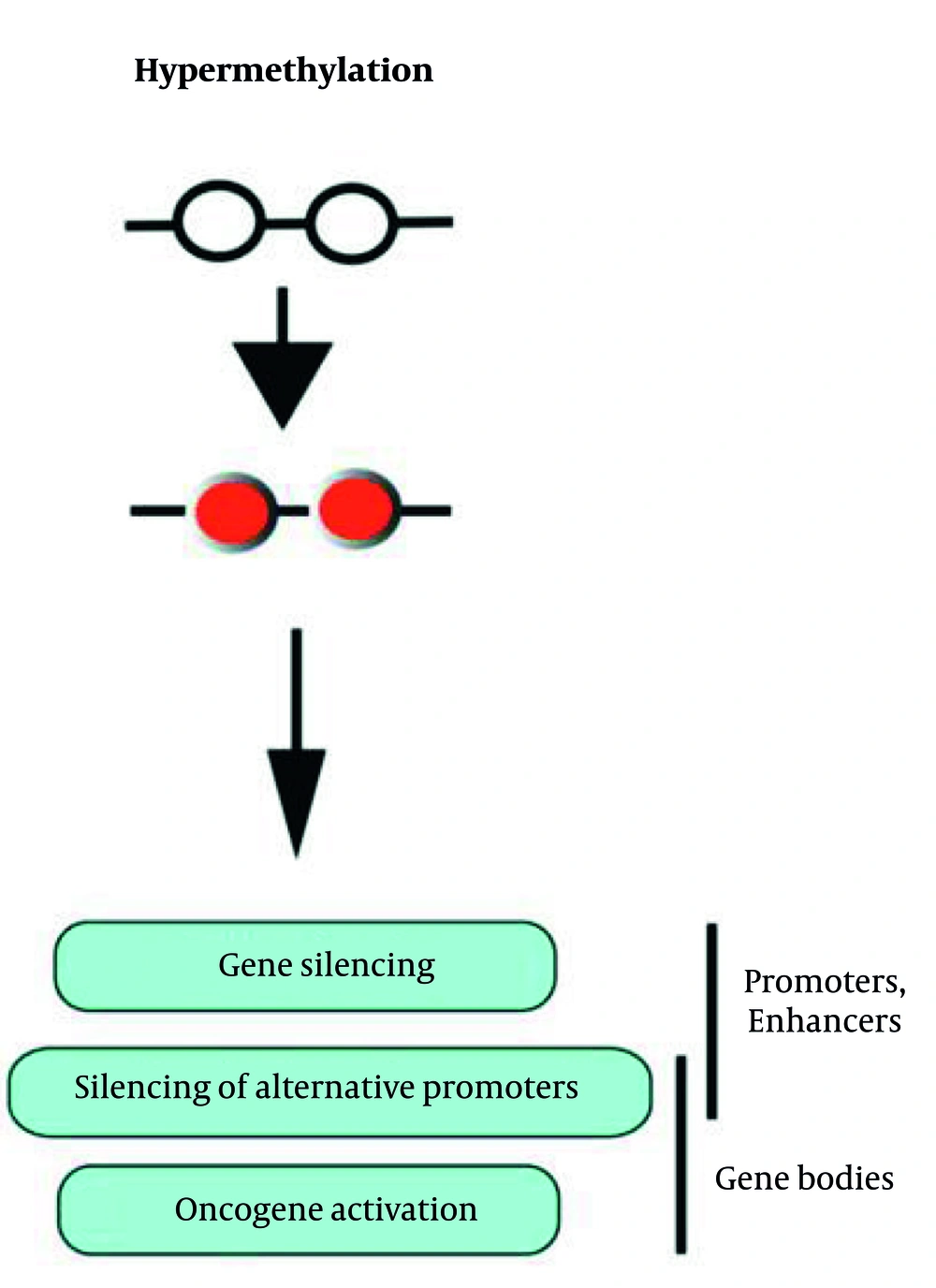
From single nucleotide polymorphism (SNP) analysis, three loci associated with uterine fibroids have been identified. Additionally, DNA methylation and histone modification can be inherited and independently regulate gene expression from the primary DNA sequence. DNA methyltransferase acts as a catalyst, facilitating the attachment of methyl groups to cytosines in cytosine-guanine (CpG) sequences. Increased methylation in promoter regions is associated with decreased gene expression, while methylation within gene bodies is linked to increased oncogenic activity (Figure 4) (23, 24).
Hypermethylation and its effects on promoters and gene bodies (24)
Many tumor suppressors, such as the gene encoding the transcription factor KLF11, are hypermethylated and suppressed. KLF11 is also a target of progesterone or anti-progestin and plays a significant role in the growth and development of uterine fibroids (15).
Cytogenetic abnormalities indicate different genetic pathways. Although the karyotype of most uterine fibroids is normal, further studies have shown that about 50% of tumors exhibit chromosomal abnormalities. These abnormalities include alterations in chromosomes 3, 6, 7, and 13; trisomy 12; reciprocal translocation between chromosomes 12 and 14; as well as monosomy 2 (18).
3.3. Prevention and Treatment of Uterine Fibroids
Consumption of green fruits, vegetables, vitamin D, and low-fat dairy products is recommended to reduce the risk of developing uterine fibroids, although elevated levels of progesterone and estrogen can also contribute. This is because cells exposed to estrogen and progesterone produce mitogenic substances that promote the proliferation of immature neighboring cells (8, 25). Avoiding smoking, maintaining a healthy diet, and controlling blood pressure also help reduce the risk. Various treatments are used depending on the patient's condition to manage uterine fibroids. These include surgical methods such as hysterectomy with or without ovary preservation, specialized ultrasonic surgeries like MRI-guided focused ultrasound surgery (FUS), radiofrequency ablation (RFA), drug therapy, hormone therapy, and others.
3.4. Types of Uterine Fibroid Treatments
3.4.1. Hysterectomy
Hysterectomy is a relatively common surgical procedure that involves the removal of the uterus and, in some cases, the cervix. In some types of hysterectomy, the ovaries and fallopian tubes are also removed. This surgery can be performed via different approaches such as abdominal, vaginal, or laparoscopic hysterectomy (26).
3.4.2. MRI-guided Focused Ultrasound Surgery
First, precise localization of the target structure is done using MRI, which increases treatment efficiency and reduces damage and side effects to normal tissues. Then, ultrasonic waves are directed at the localized myometrial nodules to induce coagulation. This causes necrosis in tumor cells and is a non-invasive treatment protocol (27, 28).
3.4.3. Radiofrequency Ablation
Radio waves (RF) have the longest wavelength and lowest frequency, resulting in low energy, making them suitable for therapeutic protocols. A generator produces these waves, which are delivered to the target tissue via electrodes (1). This treatment method is minimally invasive with acceptable outcomes and is one of the best options for women who wish to conceive after treatment (29).
3.4.4. Drug Therapy and Hormone Therapy
Before various surgeries and other invasive methods, drug or hormone therapies are used to treat uterine fibroids. These include progesterone or progestogens, combined estrogen-progestin therapy, gonadotropin receptor blockers, gonadotropin analogs, and more. Each targets abnormal tissue in different ways (30).
3.4.5. Novel Treatments
Recent research focuses on using DNA methylation inhibitors and chromatin modulators to suppress fibroid growth. Some of these drugs have shown promising results in early clinical trials.
4. Conclusions
Given the very high prevalence of uterine fibroids, it is expected that all cellular and molecular mechanisms of this disease would be identified and understood. However, due to the complexity and multitude of causes, many unknowns still remain. As our knowledge expands regarding the cellular, genetic, immune, and epigenetic mechanisms involved in uterine fibroids, new and less invasive therapeutic prospects emerge. The use of advanced technologies such as single-cell RNA sequencing (scRNA-seq) and RNA-based therapies may provide more personalized and effective treatments in the future. Since developing therapies based on cellular and molecular sciences offers higher precision and efficiency, scientists and researchers should continue to advance in this field and apply their study results to therapeutic sciences. It is hoped that the outcomes of these studies and future research will help improve patient conditions and enhance women’s health.