1. Background
In recent years, there has been a significant increase in the clinical detection of early missed abortion (MA) cases. The incidence of early MA has dramatically risen in the past few years and currently stands at 15% (1). The MA (2) — previously termed “expired abortion” or “stillbirth” — occurs when a nonviable embryo remains in the uterus for ≥ 2 months after developmental cessation. Medical abortion is commonly practiced, with mifepristone combined with misoprostol being frequently used drugs (3, 4). Although this combination demonstrates some efficacy in inducing labor, it presents drawbacks such as incomplete abortion and excessive vaginal bleeding following an extended period of time since conception (5, 6), thereby increasing the risk of uterine infection, residual calcification, and infertility. Thus, exploring novel combined treatments is critical. Traditional Chinese Medicine (TCM) has a long history of safe application in gynecological care (7). Xinshenghua granule is a TCM preparation composed of peach kernels, safflowers, motherwort, and other ingredients. It possesses pharmacological effects that promote blood circulation and alleviate scarlet fever (8). In previous studies, “Xinshenghua” granule, a compound preparation of TCM, has been shown to enhance uterine contraction and facilitate the shedding and discharge of uterine cavity residues (9). “Fujiu No.2” is a TCM formula developed by our hospital. Previous clinical applications have demonstrated its ability to promote uterine involution and prevent adhesion in the uterine cavity. However, there is limited research on the clinical efficacy of combining Xinshenghua granule with Fujiu No.2 in the treatment of MA. Therefore, this study aimed to investigate the therapeutic effects of different medical treatment regimens for MA by selecting 218 patients admitted to our hospital from December 2018 to December 2022 as research subjects.
2. Objectives
The objective was to observe the effects of TCM treatment (Xinshenghua granule combined with Fujiu No.2) in combination with mifepristone and misoprostol on vaginal bleeding after abortion, pregnancy discharge rate, and serum hormone levels.
3. Methods
3.1. Participants
This study complied with the Helsinki Declaration. Written informed consent was obtained from all participants, and patient confidentiality was strictly maintained. A retrospective analysis was performed on 218 patients diagnosed with MA who were admitted to the Department of Obstetrics and Gynecology at Ningbo Women and Children's Hospital (December 1, 2018 - December 1, 2022). Patients were divided into an observation group (TCM combined treatment) and a control group (conventional treatment) based on their documented treatment choices in medical records (no randomization, as this was a retrospective study).
The inclusion criteria for patients in this study are as follows: (1) Confirmed MA via transvaginal ultrasound: No embryonic cardiac activity, gestational sac size inconsistent with gestational age (no growth for ≥7 days), and/or intrauterine hematoma (when applicable); (2) gestational age of 6 - 16 weeks (calculated via last menstrual period and ultrasound biometry; discrepancies resolved by ultrasound); (3) no history of tocolytic use (e.g., magnesium sulfate, ritodrine) within 3 months before admission; (4) no history of adverse pregnancy outcomes (e.g., recurrent miscarriage, stillbirth) or familial genetic diseases (e.g., chromosomal abnormalities confirmed by prior prenatal testing); (5) voluntarily request for medical abortion and provision of written informed consent.
The exclusion criteria are: (1) Contraindications to mifepristone/misoprostol: Severe liver/kidney dysfunction (alanine transaminase > 3 × upper limit of normal, serum creatinine > 1.5 × upper limit of normal), coagulation disorders (prothrombin time > 13 seconds), or uncontrolled hypertension; (2) ectopic pregnancy (confirmed by ultrasound and serial serum β-human chorionic gonadotropin (β-HCG) monitoring: < 66% increase in 48 h); (3) intrauterine device (IUD) in place during pregnancy (confirmed by ultrasound); (4) genital tract malformations (e.g., uterine septum, bicornuate uterus) or acute genital inflammation (e.g., acute cervicitis, pelvic inflammatory disease with fever > 38℃); (5) history of neurological/psychiatric conditions (e.g., epilepsy, severe depression) that could affect treatment adherence or outcome assessment; (6) documented allergies to any component of mifepristone, misoprostol, Xinshenghua granule (e.g., Angelica sinensis), or Fujiu No.2 (e.g., borneol).
Sample size calculation was performed a priori: Assuming a 10% loss to follow-up, a sample size of 133 (66 - 67 per group) was required to achieve 90% power (2-tailed, α = 0.05) for detecting a 20% difference in pregnancy tissue expulsion time (primary outcome) between groups.
3.2. Treatment Technique
All patients received mifepristone and misoprostol for pregnancy termination.
Day 1: Oral mifepristone (150 mg, empty stomach, 2 h before meals).
Day 2: Oral misoprostol (50 μg, with food to reduce gastrointestinal side effects).
Day 3: Oral misoprostol (600 μg); an additional 600 μg was administered if pregnancy tissue was not expelled within 3 h (under close monitoring for uterine contractions).
The control group received only the above conventional therapy. The observation group, in addition to conventional therapy, received:
1. Xinshenghua granule: Composed of A. sinensis, Ligusticum chuanxiong, ginger charcoal, peach kernel, fried licorice, motherwort, and safflower. Dosage: 12 g per dose, 3 times daily (orally, after meals) for 3 days.
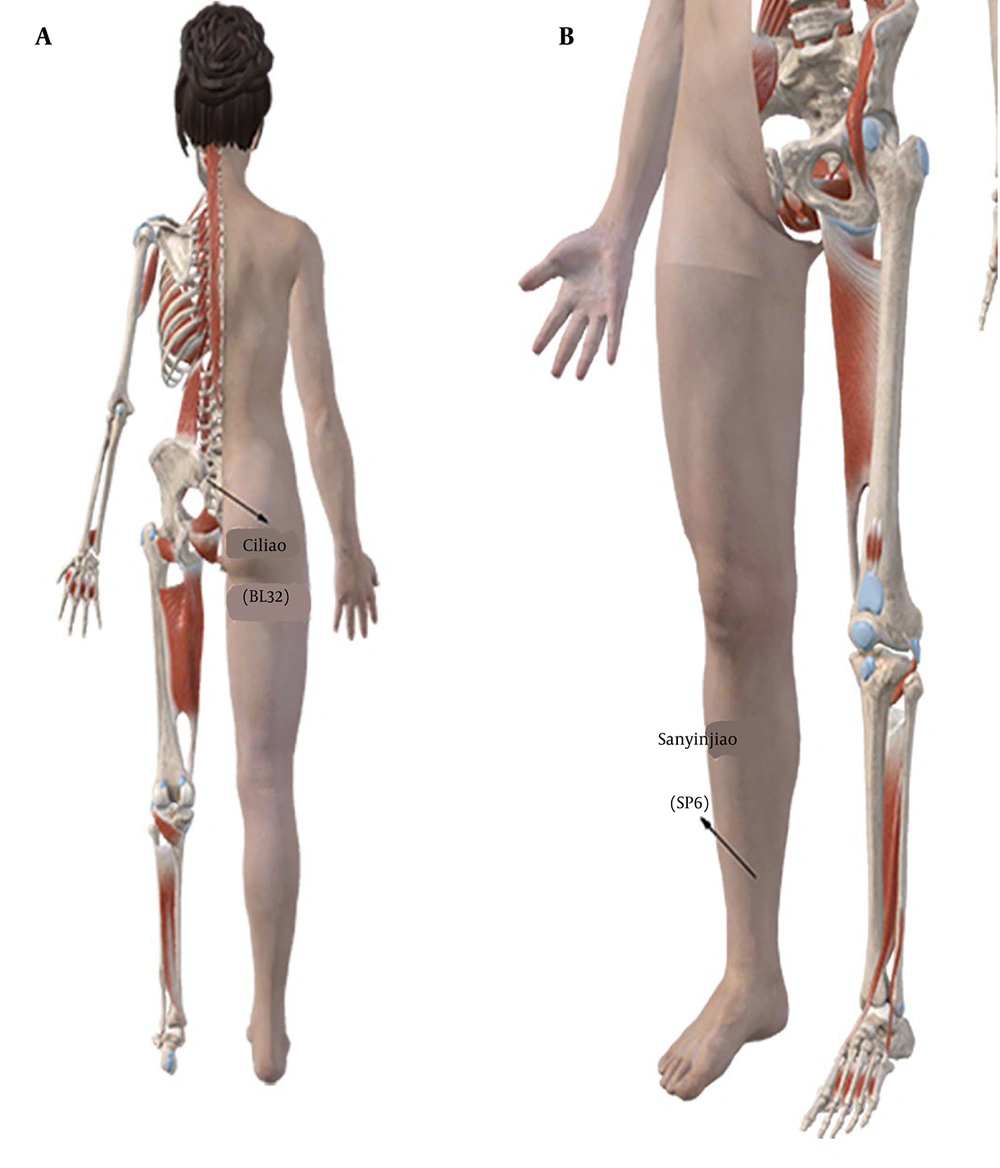
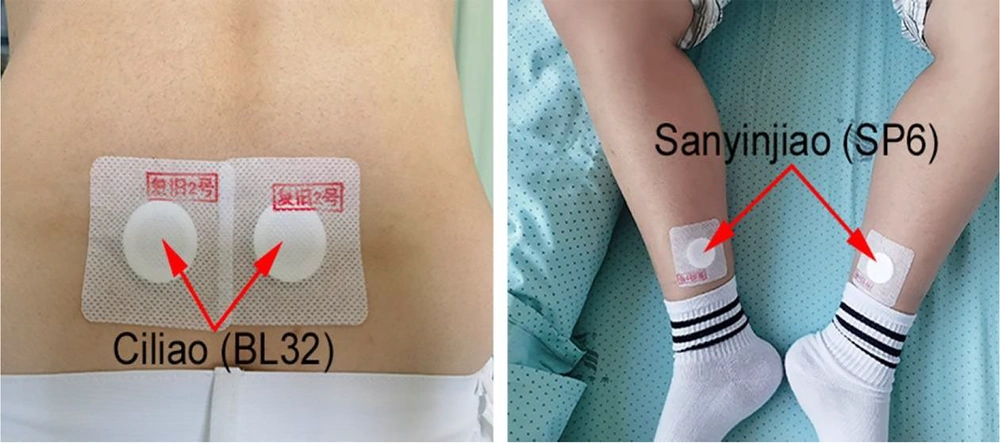
2. “Fujiu No.2” formula (Figure 1) and its Ingredients in our hospital are as follows: Angelica sinensis (24 g, Figure 1A), L. chuanxiong (9 g, Figure 1B), peach kernel (6 g, Figure 1C), dried ginger (2 g, Figure 1D), borneol (2 g, Figure 1E), red peony (6 g, Figure 1F), etc. Preparation method: Powder the aforementioned herbs, mix them with petroleum jelly, form them into treatment pills, and apply them to the Ciliao (BL32) and Sanyinjiao (SP6) acupoints (Figures 2 and 3). Acupoint application method: Once daily, 6 h per session (removed if skin irritation occurred) for 3 days. Both groups were treated for three days. Curettage was performed if excessive bleeding occurred (> 100 mL in 1 hour) after placental tissue removal. Ciliao (BL32, Figure 2A) and Sanyinjiao (SP6, Figure 2B) acupoint location: Ciliao [BL32, Bladder Meridian: Over the second sacral foramen, regulates gynecological function (10)] and Sanyinjiao [SP6, Spleen Meridian: Three cun above the medial malleolus, stimulates uterine activity (11)].
Chinese herbal formula of Fujiu No.2: The Traditional Chinese Medicine (TCM) ingredients contained in the newly developed biochemical granules include: A, Angelica sinensis (24 g); B, Ligusticum chuanxiong (9 g); C, peach kernel (6 g); D, dried ginger (2 g); E, borneol (2 g), and red peony. The Xinshenghua granule is a formulation composed of the aforementioned TCM ingredients, developed and utilized by our hospital. The same composition of TCM ingredients is administered to each patient following surgical procedures.
3.3. Outcome Measurements
1. Comparison of clinical outcomes between the two groups: Focused on pregnancy tissue expulsion time, which is defined as the duration from the first dose of mifepristone to confirmation of no intrauterine residual tissue via transvaginal ultrasound (residue < 5 mm was considered “no residual”).
2. Evaluation of vaginal bleeding volume and duration within 24 h post-expulsion of pregnancy tissue: Vaginal bleeding volume was measured via the gravimetric method with standardized sanitary pads (1 g weight increase = 0.95 mL blood, based on blood specific gravity of 1.05). The duration of vaginal bleeding was defined as the period from the first dose of medication to complete cessation of visible vaginal bleeding (confirmed by no blood stains on pads for 24 consecutive hours).
3. Hospitalization duration: Calculated from the admission date to the discharge date (discharge criteria: No residual tissue, stable vital signs, no active bleeding).
4. Comparison of serum β-HCG levels before and after treatment in both groups: Fasting venous blood samples (3 mL) were collected pre-treatment (day 1) and post-treatment (3 days after treatment, day 4). Serum was separated by centrifugation (3000 rpm, 10 minutes), and β-HCG was measured via chemiluminescent immunoassay (detection range: 0.1 - 100,000 mIU/mL).
Binary logistic regression was used to assess treatment effects. The model compared an experimental group receiving TCM combination therapy with a control group not receiving TCM treatment. Covariates included age, height, weight, gravidity and parity, vaginal bleeding volume, vaginal bleeding duration, menstrual interruption time, pregnancy material discharge time, and changes in HCG levels prenatally and postpartum. Regression analysis results were reported as odds ratios (ORs) with 95% confidence intervals (CIs) to minimize the risk of false-positive findings. A P-value of less than 0.05 was considered statistically significant. All data analyses were conducted using SPSS version 27. Outliers (identified via box plots) were retained if biologically plausible (confirmed via medical records) and their exclusion did not alter primary results.
4. Results
The clinical characteristics of the 218 patients are detailed in Table 1. The final study included 108 patients in the observation group and 110 in the control group (no loss to follow-up). There were no significant differences in baseline characteristics between groups (all P > 0.05, Table 1). The mean age of the experimental group was 27.86 ± 4.535 years, compared to 27.87 ± 4.450 years in the control group. The average height was 160.34 ± 5.02 cm for the experimental group and 160.15 ± 5.309 cm for the control group. The mean weight was 56.071 ± 8.489 kg for the experimental group and 55.267 ± 9.261 kg for the control group. Additionally, 50.92% of the experimental group and 42.7% of the control group were primiparas. There were no statistically significant differences between the two groups in terms of age, height, weight, gravidity, or parity (P > 0.05).
| Characteristics | Cases (N = 108) | Controls (N = 110) | F/χ2 | P-Value (95%CI) |
|---|---|---|---|---|
| Age b | 27.86 ± 4.535 | 27.87 ± 4.450 | 0.009 | 0.985 (-1.211, 1.188) |
| Weight b (kg) | 56.071 ± 8.489 | 55.267 ± 9.261 | 0.250 | 0.505 (-1.5671, 3.1752) |
| Height b (cm) | 160.34 ± 5.02 | 160.15 ± 5.309 | 0.098 | 0.788 (-1.191, 1.567) |
| Gravidity c | 2.910 | 0.714 | ||
| 0 | 55 (25.2) | 47 (21.6) | ||
| 1 | 28 (12.8) | 36 (16.5) | ||
| 2 | 11 (5) | 15 (6.9) | ||
| 3 | 10 (4.6) | 10 (4.6) | ||
| 4 | 2 (0.9) | 1 (0.5) | ||
| 5 | 2 (0.9) | 1 (0.5) | ||
| Parity c | 1.409 | 0.235 | ||
| 0 | 107 (49.) | 110 (50.5) | ||
| 1 | 0 | 0 | ||
| 2 | 1 (0.5) | 0 | ||
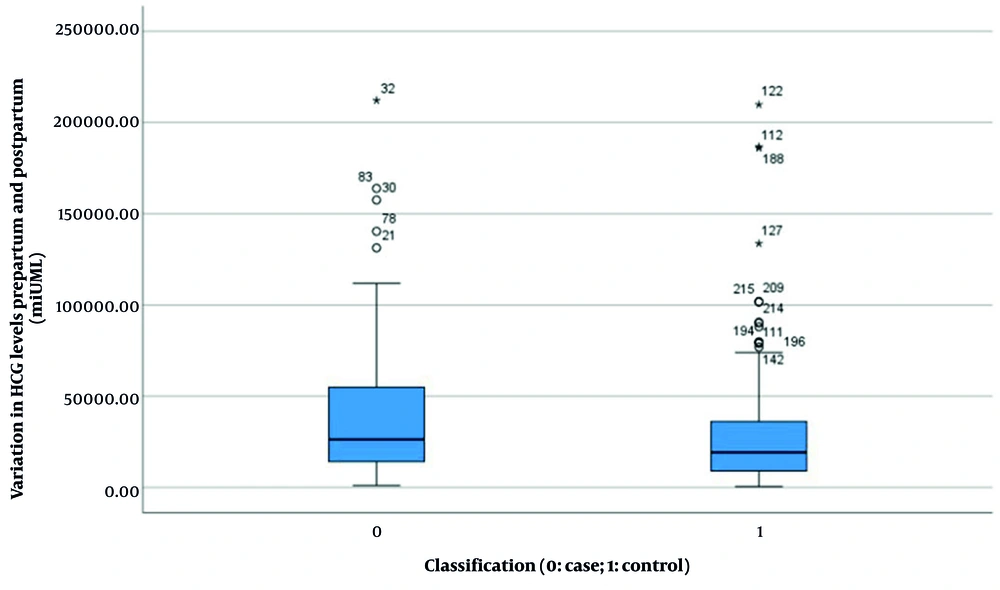
| Variation in HCG levels prepartum and postpartum (mIU/ML) b | 38955.397 ± 37183.494 | 31909.021 ± 37958.554 | 0.192 | 0.168 (-2984.6, 17077.36) |
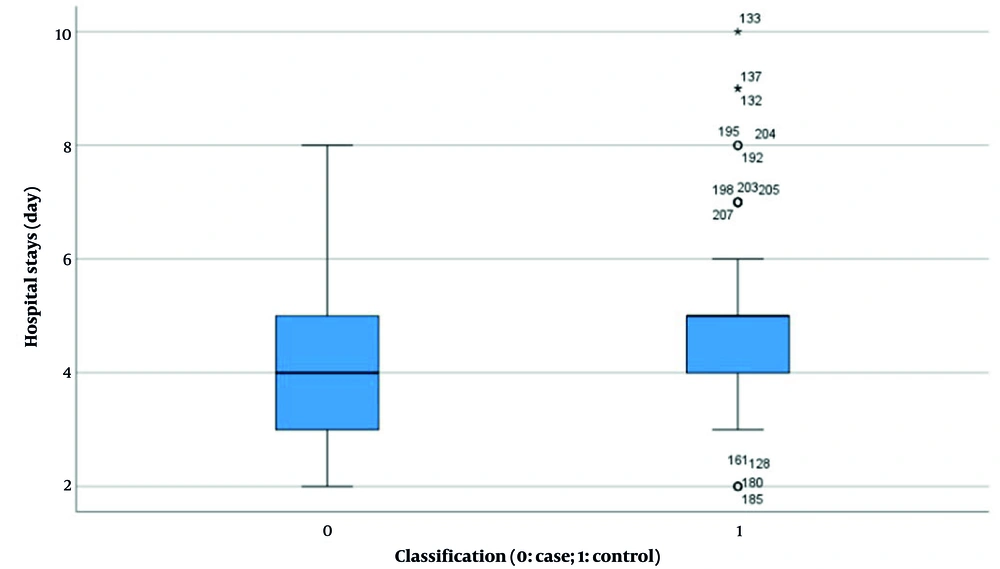
| Hospital stays (d) b | 4.340 ± 1.185 | 4.950 ± 1.426 | 0.095 | < 0.001 (-0.953, -0.253) |
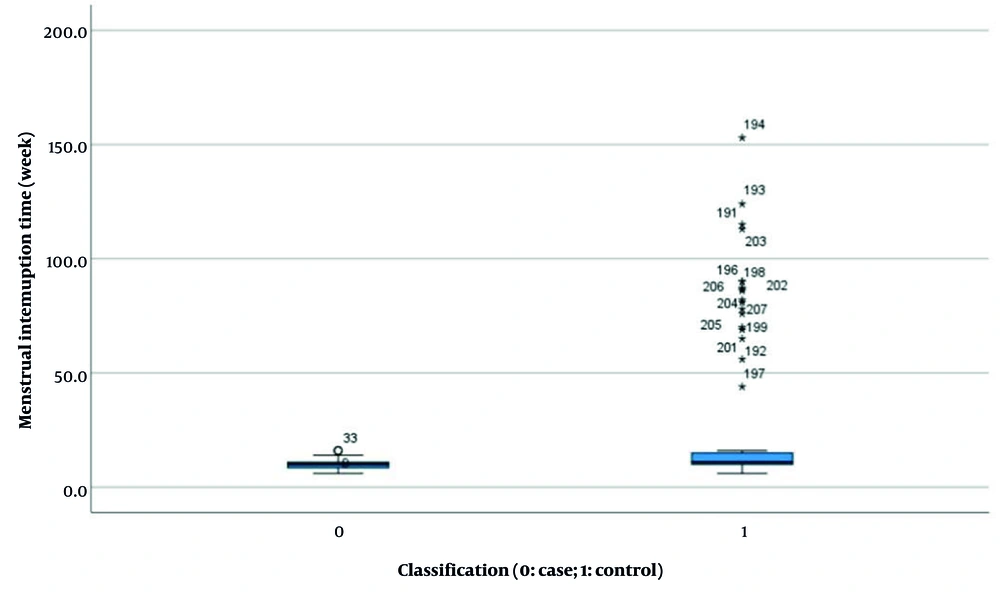
| Menstrual interruption time (wk) b | 9.824 ± 1.766 | 23.559 ± 30.056 | 86.562 | < 0.001 (-19.424, -8.046) |
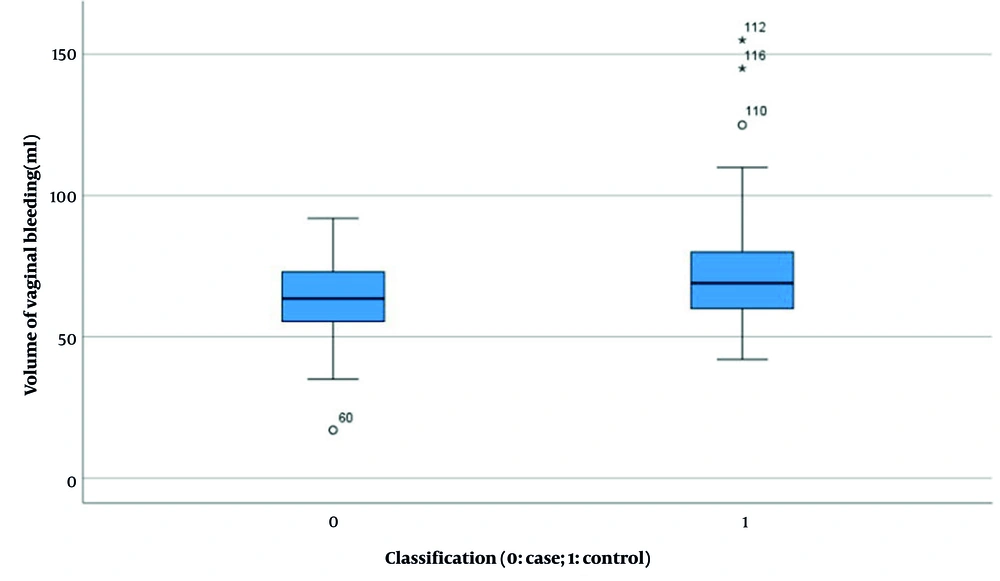
| Volume of vaginal bleeding (mL) b | 63.52 ± 12.889 | 72.8 ± 19.250 | 7.307 | < 0.001 (-13.633, -4.9) |
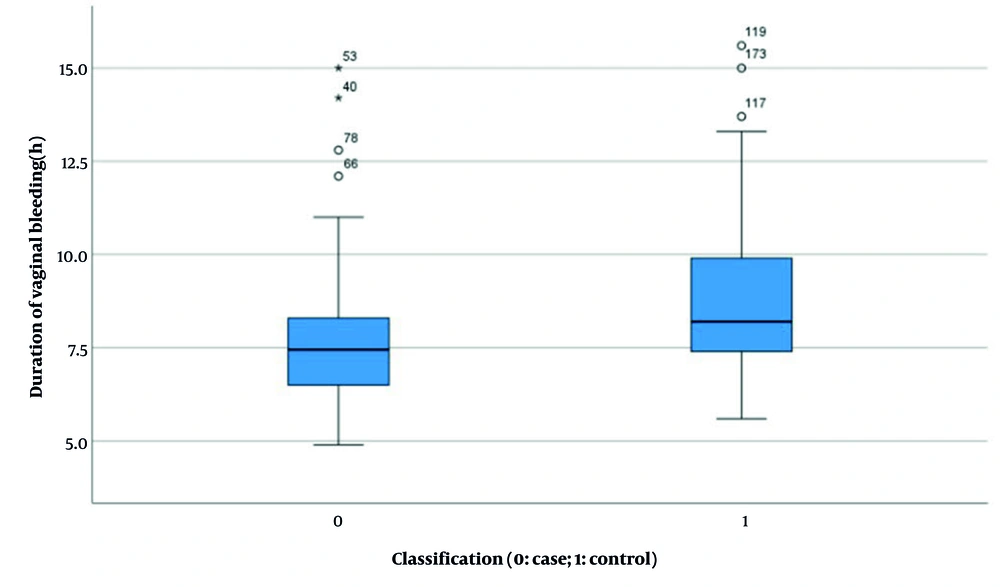
| Duration of vaginal bleeding (h) b | 7.632 ± 1.767 | 8.728 ± 1.975 | 2.504 | < 0.001 (-1.596, -0.596) |
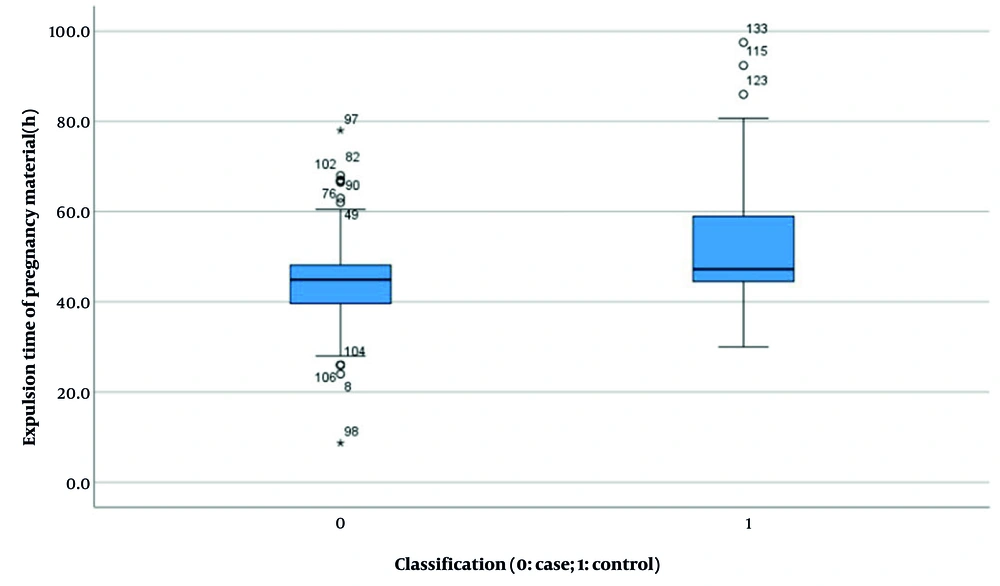
| Expulsion time of pregnancy material (h) b | 44.510 ± 10.402 | 52.077 ± 12.915 | 8.139 | < 0.001 (-10.696, -4.439) |
Abbreviation: CI, confidence interval.
a Values are expressed as mean ± SD or No. (%).
b For difference between case and control, the test was performed by using independent-samples t-test.
c Chi-square test was used for categorical variables.
Results indicated no significant difference in HCG levels between the two groups before and after pregnancy (experimental group: 38955.397 ± 37183.494 vs. control group: 31909.021 ± 37958.554, P = 0.168, Figure 4). However, statistically significant differences were observed in several parameters: Length of hospital stay (4.340 ± 1.185 days vs. 4.950 ± 1.426 days, P < 0.001, Figure 5), duration of menstrual interruption (9.824 ± 1.766 days vs. 23.559 ± 30.056 days, P < 0.001, Figure 6), vaginal bleeding time (7.632 ± 1.767 days vs. 8.728 ± 1.975 days, P < 0.001, Figure 7), vaginal bleeding volume (63.52 ± 12.889 mL vs. 72.8 ± 19.250 mL, P < 0.001, Figure 8), and discharge time of pregnancy objects (44.510 ± 10.402 h vs. 52.077 ± 12.915 h, P < 0.001, Figure 9).
Prior to statistical analysis, the dataset was examined for potential outliers using box plot analysis. Although several potential outliers were identified in the HCG levels, length of hospital stay, duration of menstrual interruption, vaginal bleeding time, vaginal bleeding volume, and discharge time of pregnancy objects group, they were retained in all subsequent analyses for the following reasons: First, they were deemed to be legitimate and biologically plausible values upon reviewing the raw data and experimental conditions; second, their exclusion did not qualitatively alter the statistical significance of the primary findings.
After adjusting for confounding factors, the clinical efficacy between the experimental group and the control group was compared. In accordance with previous studies, a multivariate logistic regression analysis was performed to evaluate clinical outcomes. The analysis incorporated variables including demographic characteristics (age, height, weight) and reproductive history (including number of pregnancies, number of times pregnant, and number of births). Additionally, clinical features such as volume and duration of vaginal bleeding, timing of menopause, expulsion time of pregnancy material, and changes in HCG levels pre- and post-delivery were also considered. The results related to efficacy after excluding the influence of confounding factors are shown in Table 2.
| Variables | B | Standard Error | Wald | OR | P-Value |
|---|---|---|---|---|---|
| Age | -0.28 | 0.049 | 0.337 | 0.972 | 0.562 |
| Hospital stays | -0.266 | 0.202 | 1.728 | 0.766 | 0.189 |
| Menstrual interruption time | 0.374 | 0.097 | 14.753 | 1.454 | < 0.001 |
| Gravidity | 0.243 | 0.192 | 1.613 | 1.276 | 0.204 |
| Parity | -9.569 | 0.000 | 0.000 | 0.000 | 1 |
| Weight | 0.006 | 0.024 | 0.073 | 1.006 | 0.788 |
| Height | -0.009 | 0.042 | 0.049 | 0.991 | 0.824 |
| HCG levels pre- and post-delivery | 0.000 | 0.000 | 10.178 | 1.0 | 0.001 |
| Volume of vaginal bleeding | -0.006 | 0.012 | 0.231 | 0.994 | 0.631 |
| Duration of vaginal bleeding | 0.441 | 0.112 | 15.551 | 1.554 | < 0.001 |
| Expulsion time of pregnancy material | 0.071 | 0.021 | 12.058 | 1.074 | < 0.001 |
Abbreviation: OR, odds ratio.
After adjusting for confounding factors, the final analysis revealed statistically significant differences between the two groups with respect to menstrual interruption duration, changes in HCG levels before and after delivery, duration of vaginal bleeding, and expulsion time of pregnancy tissue.
5. Discussion
The MA is a condition characterized by the retention of a nonviable fetus in the uterus for an extended period due to various factors, accompanied by the disappearance of early pregnancy symptoms and the cessation of uterine growth (12). The retained fetal tissue often adheres tightly to the uterine wall, complicating its removal and increasing the risk of significant hemorrhage (13). Furthermore, the progressive release of lysosomal enzymes from fetal visceral autolysis can exacerbate maternal coagulation disorders, potentially leading to severe maternal morbidity or mortality (14, 15). Therefore, prompt diagnosis and immediate intervention are imperative.
Historically, the combination of mifepristone and misoprostol has been used in clinical settings for the management of MA (16). Mifepristone, a receptor-level antiprogestin, selectively binds to decidual progesterone receptors, competing with endogenous progesterone and thereby inhibiting its effects. This results in decidual and villous tissue degeneration, reduced cell proliferation, and eventual necrosis due to compromised blood supply. Misoprostol (17), a prostaglandin E1 analog, facilitates cervical dilation and uterine contractions, aiding in the expulsion of pregnancy remnants. The synergistic action of these two medications enhances the detachment and expulsion of the organized embryonic tissue from the uterine lining (18). However, long-term clinical experience has shown that while this regimen is effective, it is associated with a high incidence of incomplete abortion, prolonged bleeding, infection, and the need for subsequent surgical curettage, which carries significant procedural risks and potential harm to the patient.
The TCM boasts a long history and extensive experience in external treatments, reflecting the accumulated wisdom of humanity. One such external TCM therapy, the topical application to acupoints, has developed a sophisticated theoretical framework through the contributions of historical experts. Modern research indicates that this method can bypass the first-pass metabolism in the liver and reduce gastrointestinal irritation, thereby enhancing the bioavailability of herbal medicines. Grounded in the principles of TCM efficacy and meridian theory, this technique stimulates acupoints, exerts regulatory effects, and maximizes the therapeutic potential of TCM while facilitating the synergistic interaction between different herbs. Consequently, it helps to regulate qi and blood, harmonize yin and yang, and is widely applied in the treatment of various conditions (19).
Ciliao (BL32) acupoints are widely employed in the treatment of gynecological and reproductive disorders (10). Sanyinjiao acupoints (SP6) primarily influence labor by modulating neural reflex mechanisms, thus stimulating uterine activity (11). Similarly, Sanyinjiao (SP6) and Ciliao (BL32) acupoints are also widely utilized in the treatment of male patients. The combined application of these two acupoints has been found to enhance therapeutic outcomes, particularly in the management of chronic prostatitis (20) and sexual dysfunction (21) in male patients.
According to TCM, the primary causes of MA encompass dietary indiscretion, emotional trauma, excessive exertion, and physical weakness. Xinshenghua granule is a TCM compound preparation composed of A. sinensis, motherwort, chuanxiong, Radix bupleurum, and other herbal ingredients. Angelica sinensis nourishes blood and promotes circulation while invigorating the spleen and qi. Motherwort facilitates blood circulation and resolves blood stasis, aiding in postpartum recovery. Chuanxiong activates blood circulation, resolves blood stasis, and regulates qi to assist in fetal expulsion. Bupleurum harmonizes the exterior and interior, soothes the liver, and alleviates depression. The synergistic effects of these herbs can warm the meridians, promote blood circulation, resolve blood stasis, and generate new vitality, thereby achieving multiple therapeutic benefits such as resolving blood stasis, analgesia, hemostasis, and accelerating postpartum recovery. Chinese herbal medicine has been utilized in Asia for centuries and can effectively treat MA with minimal side effects (19); therefore, a Chinese herbal compound preparation was employed in our clinical study protocol.
"Fujiu 2" is an in-house TCM formula at our hospital, comprising ingredients such as angelica, notoginseng, peach kernel, ginger, borneol, and red peony root. Angelica and notoginseng nourish and invigorate the blood, promoting circulation and preventing stagnation. Peach kernel facilitates blood flow and alleviates pain. Borneol and red peony root clear heat, detoxify, resolve stasis, and relieve pain. When applied to acupoints like Ciliao and Sanyinjiao, these herbs effectively promote uterine regeneration and prevent intrauterine adhesions.
This study demonstrated that adding TCM (Xinshenghua granule+Fujiu No.2) to conventional therapy significantly improved outcomes. The observation group had faster pregnancy tissue expulsion (44.51 vs. 52.08 h), shorter vaginal bleeding (7.63 vs. 8.73 days), less bleeding volume (63.52 vs. 72.80 mL), and superior β-HCG recovery (all P < 0.05). These benefits are attributed to the mechanisms of the TCM interventions.
Xinshenghua granule: Angelica sinensis nourishes blood and promotes circulation; motherwort resolves blood stasis; L. chuanxiong regulates qi — collectively enhancing uterine contraction and residue discharge.
Fujiu No.2 acupoint application: Applied to Ciliao (BL32) and Sanyinjiao (SP6), this intervention bypasses hepatic first-pass metabolism (increasing herbal bioavailability) and stimulates acupoints to promote uterine involution and prevent adhesion.
Multivariate regression confirmed that TCM therapy independently improved key outcomes (menstrual interruption time, β-HCG recovery, bleeding duration) after adjusting for baseline factors — supporting its efficacy beyond conventional therapy. Notably, these findings align with prior research showing TCM’s safety in reducing MA complications (7, 9), but future studies should explore long-term outcomes (e.g., subsequent pregnancy) to further validate the regimen.
In summary, the clinical efficacy of Xinshenghua granule in combination with Fujiu No. 2 for managing MA is superior to that of conventional uterine contraction therapy. This treatment regimen is characterized by reduced vaginal bleeding, accelerated expulsion of pregnancy tissues, and improved restoration of serum hormone levels following treatment. In conclusion, combining Xinshenghua granule and Fujiu No.2 with mifepristone-misoprostol therapy significantly improves MA management.
5.1. Limitations
This study had three main limitations:
1. As a retrospective analysis, residual confounding from unmeasured factors (e.g., psychosocial stress, dietary habits) cannot be excluded.
2. The single-center sample may limit generalizability to other populations.
3. Long-term outcomes (e.g., subsequent pregnancy rates, intrauterine adhesion incidence) were not evaluated.
Future prospective, multicenter studies are needed to validate these findings.