1. Background
Rheumatoid arthritis (RA) is a chronic, systemic autoimmune disease marked by pervasive inflammation that primarily affects multiple joints, leading to progressive polyarthritis, joint destruction, and a spectrum of systemic complications (1). Recent global epidemiological data indicate a troubling increase in the incidence and burden of RA, particularly among young adults, with a pronounced prevalence in low-income regions (2). The complex systemic nature of RA, along with alterations in serological markers, highlights the urgent need for timely and effective management strategies to alleviate the disease’s impact on patients’ quality of life (3).
Current treatment approaches for RA prominently feature disease-modifying antirheumatic drugs (DMARDs) as the cornerstone of management for newly diagnosed patients. In addition to DMARDs, the management of inflammation and prevention of joint damage often involves nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids, such as prednisolone (4, 5). However, the prolonged use of prednisolone is associated with a range of adverse systemic effects, including heightened risks of infections, osteoporosis, and metabolic derangements (6). Therefore, identifying safe and effective strategies for tapering or discontinuing corticosteroid therapy without undermining disease control is of paramount importance.
Among the various factors that may influence the management of oral prednisolone in RA patients, the presence of anti-cyclic citrullinated peptide (anti-CCP) antibodies stands out. These autoantibodies are recognized for their high specificity in diagnosing and prognosing RA (7) and have been demonstrated to correlate with disease flare-ups and extra-articular manifestations (8-10). While anti-CCP antibodies are diagnostic markers, their role in corticosteroid management remains unclear. Methotrexate (MTX) is a cornerstone therapy; however, its interaction with anti-CCP titers warrants further exploration (8-10).
We hypothesize that higher levels of baseline anti-CCP antibody titers are associated with a reduced likelihood of successfully tapering or discontinuing oral prednisolone in newly diagnosed RA patients. This relationship may be indicative of underlying disease activity and severity of inflammation, thereby influencing the management decisions made by rheumatologists.
2. Objectives
In the present study, we seek to rigorously test this hypothesis by exploring the association between anti-CCP titers and the reduction or discontinuation of prednisolone in RA patients from Mashhad, Iran.
3. Methods
3.1. Study Population
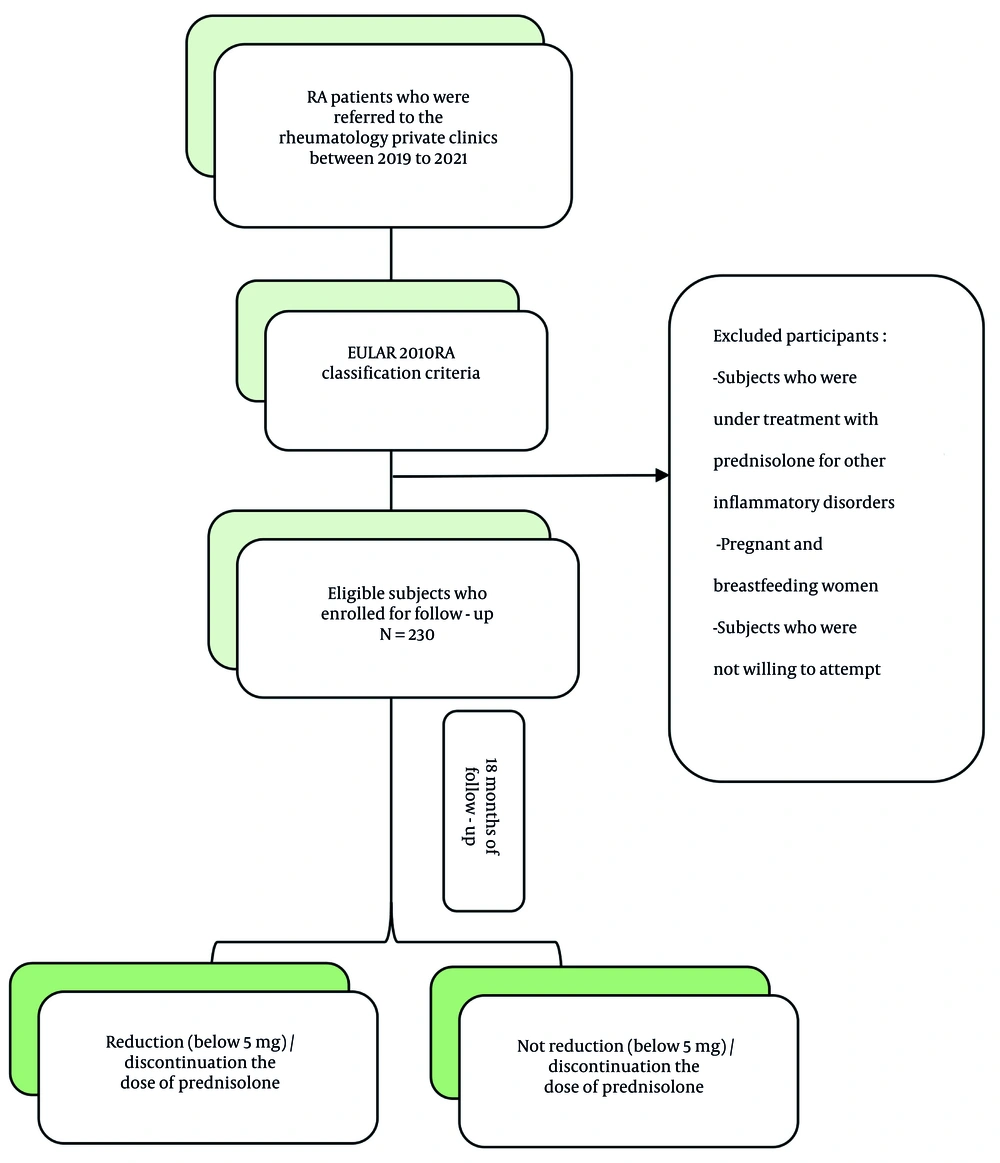
A retrospective cohort of 230 RA patients from Mashhad, Iran, was studied between 2019 and 2021. The inclusion criteria were as follows:
1. Newly diagnosed rheumatoid arthritis: Patients must have received a new diagnosis of RA according to the EULAR 2010 criteria.
2. No prior corticosteroid treatment: Subjects who were not receiving prednisolone for any other inflammatory disorders prior to enrollment.
Eligible patients were initiated on MTX, which was started at 10 - 15 mg/week and adjusted according to clinical response, and/or DMARDs, either conventional or biologic, in combination with low-dose prednisolone (5 to 10 mg). These patients were followed for 18 months. The anti-CCP titers were evaluated using enzyme-linked immunosorbent assay (ELISA, Euroimmun, Germany) at the onset of the diagnostic process. After the 18-month follow-up, patients who achieved remission were identified.
3.2. Classification of Subjects
Following the follow-up period, patients were classified based on their use of prednisolone.
1. Reduction or discontinuation group: Subjects who reduced their prednisolone dose to below 5 mg or fully discontinued it.
2. Stable group: Subjects who were unable to discontinue or reduce their prednisolone dosage despite meeting remission criteria.
Additionally, anti-CCP titers were stratified into three categories.
1. High titer: > 3 × upper limit of normal (ULN).
2. Low titer: 1 - 3 × ULN.
3. Normal titer: Within normal range (≤ 1 × ULN).
A flowchart outlining the study design is provided in Figure 1.
3.3. Operational Definitions
The diagnosis of RA was established based on the EULAR 2010 RA classification criteria, which include four classification domains with assigned point scores.
1. Joint involvement.
2. Serology: Levels of rheumatoid factor and/or anti-CCP.
3. Acute-phase reactants: C-reactive protein (CRP) and/or erythrocyte sedimentation rate (ESR). The CRP was quantified via immunoturbidimetry (Roche Diagnostics), with a normal range of < 1 mg/dL, and ESR was measured by the Westergren method.
4. Symptom duration: Symptoms lasting either < 6 weeks or > 6 weeks (10).
The remission phase was defined according to the ACR/EULAR criteria for RA remission, which encompasses:
- Number of tender joints ≤ 1.
- Number of swollen joints ≤ 1.
- The CRP ≤ 1 mg/dL.
- Global assessment score ≤ 1 (on a scale of 0 - 10).
- Alternatively, at any time point, a Simplified Disease Activity Index (SDAI) score ≤ 3.3 (11).
3.4. Ethical Considerations
All patients were thoroughly informed about the study’s objectives and procedures, and written consent for voluntary participation was obtained prior to their inclusion. The study received approval from the Ethics Committee of Mashhad Medical Sciences, Islamic Azad University, with the ethics code IR.IAU.MSHD.REC.1401.143.
3.5. Data Collection
Demographic characteristics, including age, sex, duration of symptoms, types of medications used, and patterns of prednisolone use, were collected using a standardized questionnaire. Laboratory parameters, including anti-CCP titers, were assessed from 20 mL blood samples obtained via venipuncture of an antecubital vein. The anti-CCP titer was measured using the ELISA method.
3.6. Follow-up
All participants were monitored for 18 months following the baseline examination. Regular assessments were conducted to evaluate disease activity and to record any adverse effects associated with the use of prednisolone.
3.7. Statistical Analysis
Descriptive statistics were computed, presenting means ± standard deviations for continuous variables and frequencies (%) for categorical variables. The Student’s t-test and ANOVA were utilized to compare continuous variables, while the chi-square test was employed for categorical variables to assess demographic and clinical characteristics of the study population. Logistic regression analysis was conducted to examine the association between various factors and the reduction or discontinuation of prednisolone. All analyses were performed using SPSS version 22, with a P-value < 0.05 considered statistically significant.
4. Results
After applying the inclusion criteria, we enrolled 230 new cases of RA, with a mean age of 51.71 ± 13.08 years. The cohort predominantly consisted of females (79.6%), while males represented 20.4% of the participants. Most patients (55.2%) were treated with MTX. Baseline anti-CCP titers were predominantly within the normal range for most patients (53.5%). Following an 18-month follow-up period, 84 patients (36.5%) were identified as having successfully reduced or discontinued their prednisolone therapy (Table 1).
| Characteristics | Values |
|---|---|
| Sex | |
| Female | 183 (79.6) |
| Male | 47 (20.4) |
| Age (y) | 51.71 ± 13.08 |
| Type of medication | |
| MTX | 127 (55.2) |
| MTX + conventional DMARDs | 70 (30.4) |
| Biologic DMARDs | 33 (14.3) |
| Baseline anti-CCP titer | |
| High | 68 (29.6) |
| Low | 39 (17) |
| Normal | 123 (53.5) |
| Reduction or discontinuation use of prednisolone | |
| Yes | 84 (36.5) |
| No | 146 (63.5) |
Abbreviations: MTX, methotrexate; DMARDs, disease-modifying antirheumatic drugs; anti-CCP, anti-cyclic citrullinated peptide.
a Values are expressed as No. (%) or mean ± SD.
We performed ANOVA as suggested (Table 2), comparing mean anti-CCP titers across medication groups (MTX, MTX + conventional DMARDs, biologic DMARDs). Results showed no significant differences (P = 0.421), supporting our original conclusion that anti-CCP titers did not vary by treatment class.
| Medication Group | Anti-CCP Titer (IU/mL) | P-Value b |
|---|---|---|
| MTX monotherapy | 45.2 ± 28.6 | 0.421 |
| MTX + conventional DMARDs | 48.7 ± 31.2 | - |
| Biologic DMARDs | 42.1 ± 25.9 | - |
Abbreviations: Anti-CCP, anti-cyclic citrullinated peptide; MTX, methotrexate; DMARDs, disease-modifying antirheumatic drugs.
a Values are expressed as mean ± SD.
b ANOVA.
Table 3 delineates the baseline characteristics of the study population stratified by the reduction or discontinuation of prednisolone. Significant differences were observed across multiple parameters, including age (P = 0.022), sex (P = 0.006), and type of medication used (P < 0.001). However, no statistically significant differences were detected regarding anti-CCP titers concerning the reduction or discontinuation of prednisolone across any medication classes (Table 4).
| Characteristics | Taper of Prednisolone | Non-taper of Prednisolone | P-Value |
|---|---|---|---|
| Sex | 0.006 | ||
| Female | 75 (41) | 108 (59) | |
| Male | 9 (19.1) | 38 (80.9) | |
| Age (y) | 54.30 ± 11.37 | 50.21 ± 13.79 | 0.022 |
| Anti-CCP titer | 0.763 | ||
| High | 23 (33.8) | 45 (66.2) | |
| Low | 16 (41) | 23 (59) | |
| Normal | 45 (36.6) | 78 (63.4) | |
| Type of medication | < 0.001 | ||
| MTX | 62 (48.8) | 65 (51.2) | |
| MTX + Conventional DMARDs | 20 (28.6) | 50 (71.4) | |
| Biologic DMARDs | 2 (6.1) | 31 (93.9) | |
| Duration of disease (mo) | 26.46 ± 4.58 | 25.34 ± 4.77 | 0.085 |
Abbreviations: Anti-CCP, anti-cyclic citrullinated peptide; MTX, methotrexate; DMARDs, disease-modifying antirheumatic drugs.
a Values are expressed as No. (%) or mean ± SD.
| Medication Group; Anti-CCP Titer | Tapering of Prednisolone | Non-tapering of Prednisolone | P-Value |
|---|---|---|---|
| MTX monotherapy | 0.794 | ||
| High (> 3 × ULN) | 21 (45.7) | 25 (54.3) | |
| Low (1 - 3 × ULN) | 12 (54.5) | 10 (45.5) | |
| Normal (≤ 1 × ULN) | 29 (49.2) | 30 (50.8) | |
| MTX + conventional DMARDs | 0.237 | ||
| High | 2 (12.5) | 14 (78.5) | |
| Low | 4 (26.7) | 11 (73.3) | |
| Normal | 14 (35.9) | 25 (64.1) | |
| Biologic DMARDs | 1.000 | ||
| High | 0 (0) | 6 (100) | |
| Low | 0 (0) | 2 (100) | |
| Normal | 2 (8) | 23 (92) |
Abbreviations: Anti-CCP, anti-cyclic citrullinated peptide; ULN, upper limit of normal; MTX, methotrexate; DMARDs, disease-modifying antirheumatic drugs.
a Values are expressed as No. (%).
Logistic regression analysis presented in Table 5 demonstrated a significant association between sex and the likelihood of reducing or discontinuing prednisolone. Females, designated as the reference group, exhibited a notably higher odds of reduction or discontinuation, with an odds ratio (OR) of 3.155 (P = 0.008). Age also emerged as a significant factor, correlating with a decreased likelihood of reducing or discontinuing prednisolone, reflected in an OR of 0.972 (P = 0.019). Furthermore, patients treated with MTX (as the reference group) had significantly higher odds of reducing or discontinuing prednisolone, with an OR of 3.022 (P < 0.001).
| Variables | β (Coefficient) | Adjusted OR (95% CI) | P-Value | Reference Level |
|---|---|---|---|---|
| Sex (female vs. male) | 1.149 | 3.155 (1.42 - 6.98) | 0.008 | Female |
| Age (per year increase) | -0.029 | 0.972 (0.95 - 0.99) | 0.019 | - |
| Medication type | MTX monotherapy | |||
| MTX + conventional DMARDs | -0.478 | 0.621 (0.34 - 1.12) | 0.112 | |
| Biologic DMARDs | -1.621 | 0.198 (0.08 - 0.49) | < 0.001 | |
| Anti-CCP titer | Normal (≤ 1 × ULN) | |||
| High (> 3 × ULN) | -0.123 | 0.877 (0.45 - 1.72) | 0.484 | |
| Low (1 - 3 × ULN) | -0.081 | 0.922 (0.48 - 1.77) | 0.804 |
Abbreviations: OR, odds ratio; MTX, methotrexate; DMARDs, disease-modifying antirheumatic drugs; anti-CCP, anti-cyclic citrullinated peptide; ULN, upper limit of normal.
5. Discussion
The primary objective of our retrospective cohort study was to investigate the relationship between anti-CCP titers and the reduction or discontinuation of prednisolone in patients with RA. To our knowledge, this represents the first study to examine this relationship among Iranian RA patients. Our findings indicated no significant association between baseline anti-CCP titers and the reduction or discontinuation of prednisolone after an 18-month follow-up period.
The RA is a complex inflammatory autoimmune disorder characterized by persistent synovitis, resulting in polyarthritis and an array of systemic complications (1). Although the etiology of RA is not fully understood, it is recognized as a multifactorial disease influenced by a combination of genetic predispositions, environmental factors, and immune dysregulation (12). The presence of autoantibodies, particularly rheumatoid factor and anti-CCP, is crucial in the pathogenesis of RA and in mediating its inflammatory pathways (13). The anti-CCP antibodies exhibit high specificity for RA, often appearing prior to the onset of clinical symptoms. Elevated anti-CCP titers have been shown to correlate with increased disease activity, more frequent flare-ups, and inadequate responses to standard DMARDs (8, 9, 14). Therefore, monitoring anti-CCP titers can assist clinicians in tailoring treatment strategies for optimal patient outcomes.
Long-term management of RA often involves corticosteroids, such as prednisolone, to effectively control inflammation and prevent irreversible joint damage (4, 5). However, chronic use of corticosteroids is associated with various adverse effects, including osteoporosis, avascular necrosis, increased susceptibility to infections, and gastrointestinal complications (10). These serious risks necessitate the development of well-defined strategies for tapering or discontinuing corticosteroid therapy when not clinically indicated.
Interestingly, our results demonstrated no significant relationship between baseline anti-CCP levels and the reduction or discontinuation of prednisolone, due to RA’s multifactorial pathogenesis. Disease activity (e.g., synovitis) often drives prednisolone use, independent of autoantibody levels. This aligns with a study which noted anti-CCP’s diagnostic utility but limited predictive value for treatment adjustments (15). Contrary to another study, we found no anti-CCP-prednisolone link, possibly due to differing study designs (retrospective vs. longitudinal) (16).
Several factors may account for this lack of correlation. Firstly, the multifaceted and heterogeneous nature of RA could mean that anti-CCP levels do not directly correlate with individual inflammatory responses or treatment efficacy. While anti-CCP is a vital biomarker for diagnosing RA and monitoring disease activity (17), its role as a predictor for treatment responses, specifically in relation to corticosteroid efficacy, may be limited due to the influence of various disease-modifying factors unique to each patient.
Furthermore, the timing and method of anti-CCP titer assessment in our study could impact these findings. The anti-CCP levels were evaluated at a single time point during diagnosis, potentially missing fluctuations that arise over the disease course. As RA is characterized by periods of remission and exacerbation, capturing dynamic changes in anti-CCP levels throughout follow-up may provide a clearer picture of their relationship with corticosteroid response and disease management. Future longitudinal studies that assess anti-CCP levels over time could help bridge this knowledge gap.
Our investigation also identified significant differences in demographic parameters, including age, sex, and medication type, among patients who either reduced or discontinued prednisolone. Specifically, patients treated with MTX displayed a significantly higher probability of reducing or discontinuing corticosteroid therapy compared to those on conventional or biologic DMARDs (P < 0.001). This observation may indicate that patients with a lower disease burden or fewer systemic complications are more likely to be prescribed MTX as a monotherapy. In contrast, patients with more complex disease presentations may require a combination of therapies, complicating the tapering of corticosteroids.
Moreover, MTX’s mechanisms of action — acting as an antimetabolite that inhibits cellular proliferation and exerts robust anti-inflammatory effects — may facilitate greater ease in tapering prednisolone (18, 19). Conversely, combinations of MTX with other DMARDs may lead to a more complex therapeutic landscape, necessitating the continued use of corticosteroids. While biologic DMARDs can effectively target specific inflammatory pathways, they may not provide the broad immunosuppressive effects that facilitate corticosteroid reduction compared to MTX alone (18).
5.1. Conclusions
In conclusion, our study did not find a significant relationship between baseline anti-CCP titers and the reduction or discontinuation of prednisolone in patients with RA. This lack of correlation highlights that while anti-CCP evaluation is essential for diagnosing and prognosticating RA, its role in guiding corticosteroid management decisions may be limited. Further research is necessary to clarify the influence of anti-CCP on treatment strategies in RA patients, as we continue to seek optimal methods for managing this complex disorder.
5.2. Strengths and Limitations
A key strength of this study is that it is the first to explore the relationship between anti-CCP titers and the reduction or discontinuation of prednisolone among Iranian RA patients. However, certain limitations should be acknowledged. Firstly, although the sample size was adequate, a larger cohort might enhance the reliability of our findings. Secondly, the retrospective nature of the study may introduce certain biases, limiting the interpretation of results. Lastly, while we captured anti-CCP levels at baseline, the lack of dynamic assessments throughout the follow-up period restricts our understanding of how changes in these titers might influence treatment outcomes over time.
For prospective research, we recommend larger studies with extended follow-up periods that evaluate both the timing of prednisolone reduction and the dynamic changes in anti-CCP levels, alongside other relevant biomarkers. Such studies would contribute to a nuanced understanding of the complex interplay between disease activity and therapeutic management in RA patients. Additionally, investigating the potential impact of other clinical parameters, such as patient-reported outcomes and quality of life measures, could provide further insights into treatment strategies.