1. Background
Aortic dissection (AD) represents one of the most lethal cardiovascular emergencies, characterized by a tear in the intimal layer of the aorta, leading to the separation of the wall layers and the formation of true and false lumens. Without prompt intervention, the mortality rate exceeds 50% within the first 48 hours (1). Despite advances in surgical techniques, pharmacological strategies to prevent or slow the progression of AD remain limited, highlighting an urgent need for novel therapeutic agents.
The pathogenesis of AD involves a complex interplay of multiple pathological processes. Central to this is the phenotypic switching of vascular smooth muscle cells (VSMCs) from a contractile to a synthetic state. In the healthy aorta, contractile VSMCs express high levels of markers such as smooth muscle protein 22-α (SM22α) and alpha-smooth muscle actin (α-SMA), which are crucial for maintaining vascular tone and structural integrity. Upon pathological stimulation, these cells undergo dedifferentiation, downregulating contractile proteins while upregulating synthetic markers like osteopontin (OPN), accompanied by enhanced proliferation, migration, and secretion of proteolytic enzymes. This phenotypic transition contributes significantly to extracellular matrix (ECM) degradation — particularly of elastic fibers and collagen — which weakens the aortic wall and predisposes it to dissection (2, 3).
Concomitantly, oxidative stress and inflammation play pivotal roles in AD development (4). Excessive reactive oxygen species (ROS) production impairs elastin synthesis and promotes endothelial dysfunction while activating pro-inflammatory signaling pathways. The p38 mitogen-activated protein kinase (p38/MAPK) pathway and nuclear factor-kappa B (NF-κB) are key regulators of inflammation and stress responses (5). Their activation leads to the upregulation of inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), which further accelerate VSMC phenotypic modulation, ECM remodeling, and apoptosis — ultimately facilitating aortic wall disintegration (6).
Allicin, a sulfur-containing compound derived from garlic, has garnered attention for its broad pharmacological properties, including potent antioxidant, anti-inflammatory, and cardiovascular protective effects (7). Prior studies have demonstrated that allicin administration increases superoxide dismutase (SOD) activity and reduces oxidative stress levels (8, 9). These attributes suggest that allicin may target several critical mechanisms underlying AD pathogenesis.
2. Objectives
This study proposes to explore the therapeutic potential of allicin in AD management through a comprehensive analysis of its cardiovascular protective effects. Specifically, the modulatory effects of allicin on oxidative stress parameters, VSMC phenotypic transformation, as well as key signaling pathways (including p38/MAPK and NF-κB) associated with the potential alleviation of AD progression, will be experimentally validated. These results may provide new treatment ideas for the intervention and prognosis improvement of AD.
3. Methods
3.1. Animal Models and Dosing Regimen
The AD mouse model was established by β-aminopropionitrile (BAPN) administration. The age of the mice was a key factor in BAPN modeling; only when the mice were in the early stage of rapid growth could BAPN promote the degradation of elastic fibers, leading to AD formation (10). In this experiment, 3-week-old C57BL/6J male mice were selected. A total of sixty experimental mice were randomly categorized into three groups: A control group (n = 20), a BAPN group (n = 20), and a BAPN + allicin group (n = 20). The control group received 0.2 mL of normal saline via oral gavage, while the BAPN group received 0.2 mL of BAPN (1 g/kg/day, diluted in normal saline) by oral gavage. The BAPN + allicin group received 0.2 mL of BAPN along with allicin (14 mg/kg/day, diluted in normal saline) via oral gavage (2, 11, 12). Body weight was measured weekly for all mice. Throughout the experimental period, the survival status, time of death, and cause of death were recorded. All mice were euthanized on day 28, and aortic tissue was harvested and fixed. Aortic rupture was defined as hemorrhage into the adjacent body cavity, resulting in premature death.
3.2. Histology and Immunofluorescence
The whole mouse aorta tissue samples were fixed in 4% paraformaldehyde for 48 hours, embedded in paraffin, and sliced into 4 μm thick sections. Hematoxylin and eosin (H&E) staining was performed to assess tissue morphology. The integrity of the aortic wall elastin was evaluated using elastic Verhoeff-Van Gieson (EVG) staining. Immunofluorescence was employed to examine the expression of SM22α, α-SMA, NF-κB, p38/AMPK, collagen type 1 (COL1A1), and fibronectin (FN).
3.3. In Vitro Cell Viability Assay
Mouse aortic vascular smooth muscle cells (MOVAS) were divided into three groups: Control group, platelet-derived growth factor-BB (PDGF-BB) group (20 ng/mL), and allicin treatment group (PDGF-BB 20 ng/mL + allicin 20 μM). After trypsinization, MOVAS were seeded in 96-well plates at a density of 7 × 104 cells per well. After 24 hours of incubation, different doses of allicin and PDGF-BB were added (13, 14). Cell proliferation was assessed using a cell counting kit-8 (CCK-8, Dojindo, Shanghai, China) after 24 hours of culture at 37°C in a 5% CO2 atmosphere. The VSMCs were treated with CCK-8 solution at a final concentration of 10% for 1 - 2 hours at 37°C, and absorbance was measured at 450 nm using a microplate reader (SPARK, TECAN).
3.4. In Vitro Cell Migration Assays
The VSMCs were cultured in 6-well plastic plates until reaching 80 - 90% confluence. The monolayer cells were wounded using a 1 mL pipette tip to create “scratches”, followed by washing three times with PBS to remove the non-adherent cells. The scratched VSMCs were treated with PDGF-BB, with or without allicin, for 48 hours. Cell morphology was recorded at 48 hours by photographing the wounds. The distance between the two edges of the “scratches” was measured using ImageJ, and a reduction in distance indicated cell migration.
3.5. Oxidative Stress Assessment
Blood samples were centrifuged at 3000 g for 20 minutes, and the supernatant was collected and stored at 4°C until analysis. Plasma SOD activity was determined using a SOD assay kit (Solarbio, Beijing, China) according to the manufacturer’s instructions. Plasma malondialdehyde (MDA) levels were assessed using an MDA assay kit (Solarbio, Beijing, China) following the manufacturer's instructions. The ROS levels were measured with 2’,7’-Dichlorofluorescein diacetate (DCFH-DA) according to the instructions (KeyGEN BioTECH), and then the fluorescence intensity was measured. Fluorescence images were taken with a fluorescence microscope (Olympus, Japan).
3.6. Inflammatory Reaction
Treated MOVAS were evaluated according to the manufacturer's instructions provided with the IL-6 and TNF-α kit (KeyGEN BioTECH). Absorbance values were measured using a microplate reader (Biotek Cytation5).
3.7. Western Blotting Analysis
Total protein was isolated from VSMC or mouse aortic tissue using RIPA lysis buffer. Lysates containing equal protein concentrations (determined by the BCA method; Keygen, KGA902) were prepared. Proteins were separated by SDS-PAGE and transferred to a PVDF membrane (Millipore). After blocking for 1.5 hours in 5% skim milk powder, membranes were incubated overnight at 4°C with primary antibodies, followed by incubation with secondary antibodies (1:5000) for 2 hours at room temperature. The bands were visualized using a chemiluminescence instrument (Bio-Rad ChemiDoc Touch) with Chemiluminescent HRP Substrate (Keygen, KGP116). ImageJ was used to quantify band intensity. The following primary antibodies were utilized: Anti-OPN (Proteintech, 25715-1-AP), β-actin (Servicebio, GB11001), anti-GAPDH (Keygen, KGAA002), anti-calponin (Proteintech, 13938-1-AP).
3.8. Statistical Analysis
Data were analyzed using GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA). Results are presented as mean ± standard deviation (SD). Group comparisons were performed using analysis of variance (ANOVA) and Student’s t-test or one-way ANOVA followed by Dunnett's post-hoc test, with P-values less than 0.05 considered statistically significant.
4. Results
4.1. Allicin Suppresses β-Aminopropionitrile-Induced Aortic Dissection Formation
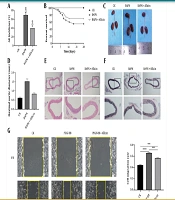
Three-week-old C57BL/6J male mice were treated with BAPN and allicin for 4 weeks. Allicin significantly reduced the incidence of AD induced by BAPN. The AD incidence in the treatment group decreased to 40%, compared to 70% in the model group (Figure 1A). Additionally, while a reduction in survival rate was observed in the BAPN group, allicin treatment restored the survival rate to 70% (Figure 1B). Moreover, BAPN induction significantly increased the maximum internal diameter of the thoracic aorta compared to the control group. Allicin notably improved the aortic morphology of BAPN-treated mice and reduced aortic dilation (Figure 1C and D). Histological examination revealed false lumen formation, destruction of the media, and marked thickening of the adventitia in the BAPN group. However, these changes were significantly attenuated by allicin treatment (Figure 1E). The elastin score of the experimental mice increased significantly after BAPN administration, and allicin treatment mitigated the breakage of elastic fibers (Figure 1F).
Allicin suppresses β-aminopropionitrile (BAPN)-Induced aortic dissection (AD) formation and inhibits mouse aortic vascular smooth muscle cell (MOVAS) migration: A, AD incidence of C57BL/6J mice in each group; B, survival curves of C57BL/6J mice in each experimental group. Control group (n = 20); BAPN group (n = 20); BAPN + allicin group (n = 20); C, macroscopic images of the aorta in each group (vehicle, BAPN, and BAPN + allicin); D, maximal aortic diameter (n = 6 per group); E, microscopic images of hematoxylin and eosin (H&E) staining of aortic sections in each group; F, microscopic images of elastic Verhoeff-Van Gieson (EVG) staining of aortic sections in each group; G, cell migration and migration rate of mouse aortic smooth muscle cells in excipients, platelet-derived growth factor-BB (PDGF-BB), and PDGF-BB + allicin (the data is expressed as mean ± standard deviation (SD); statistical significance: *** P < 0.001, and **** P < 0.0001).
4.2. Allicin Inhibits Migration of Mouse Aortic Vascular Smooth Muscle Cells
Under physiological conditions, the proliferation and migration of VSMCs are restricted. However, during endothelial injury, these capacities are abnormally enhanced, which is detrimental to cardiovascular health (15). In the migration assay, our study showed that PDGF-BB-treated VSMCs healed scratches significantly compared to the control group. Treatment with Allicin markedly inhibited PDGF-BB-induced scratch healing (Figure 1G).
4.3. Allicin Blunts Extracellular Matrix Degradation in the Aorta
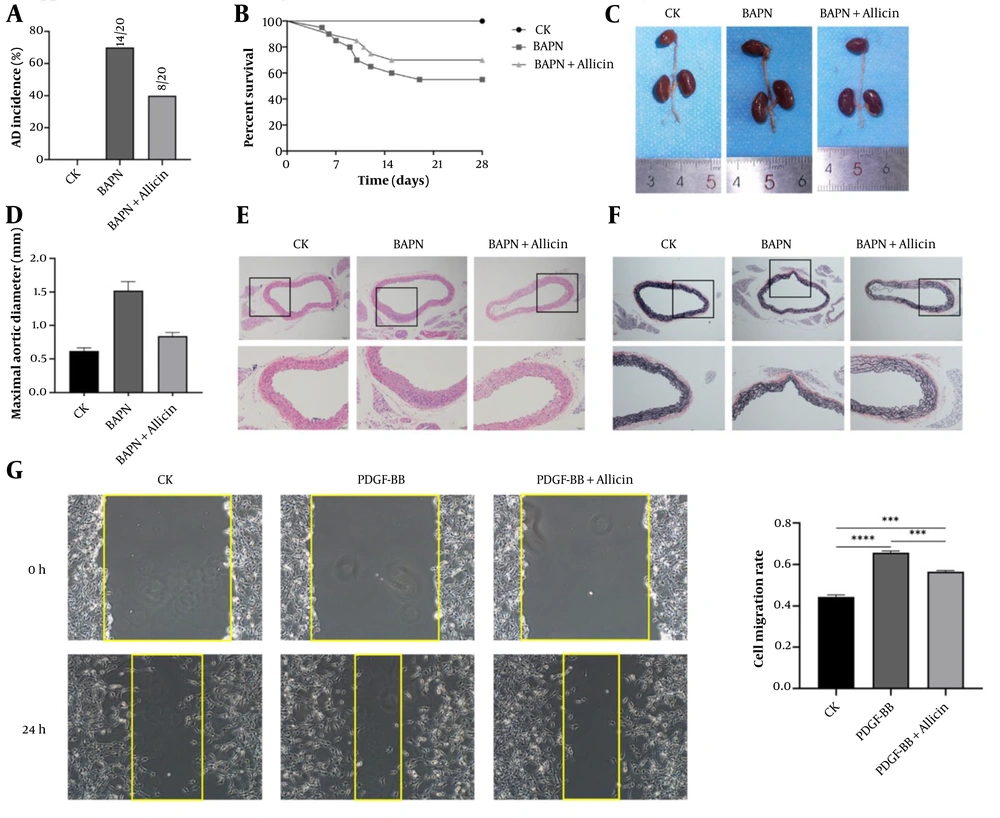
The degradation of the ECM is implicated in AD. Key ECM components, including elastic fibers, collagen fibers (e.g., COL1A1), and FN, are essential for maintaining vascular wall integrity (10). We evaluated ECM degradation in aortic lesions using immunofluorescence staining. Results showed that COL1A1 and FN levels were significantly reduced in the model group compared to controls 28 days post-modeling. Allicin treatment partially mitigated ECM degradation, as evidenced by increased COL1A1 and FN expression (Figure 2A and B).
Allicin blunts extracellular matrix (ECM) degradation of the aorta and alleviates phenotype switch of vascular smooth muscle cell (VSMC): The 3-week-old C57BL/6J male mice were treated with β-aminopropionitrile (BAPN) and allicin for 4 weeks. Mouse aortic smooth muscle cells were treated with platelet-derived growth factor-BB (PDGF-BB). A, immunofluorescence staining and quantification of collagen type 1 (COL1A1, red) in aortas of each group; B, immunofluorescence staining and quantification of Fibronectin (red) in aortas from the Vehicle, PDGF-BB, and PDGF-BB + Allicin groups; C, immunofluorescence staining and quantification of smooth muscle protein 22-α (SM22α, red) in aortas of each group; D, immunofluorescence staining and quantification of alpha-smooth muscle actin (α-SMA, red) in aortas of each group. Nuclei were stained with DAPI (blue); E, representative western blot images of SM22α and α-SMA in each group during cell experiments. Quantification of F, SM22α; and G, α-SMA; H, representative western blot images of calponin and osteopontin (OPN) in each group. Quantification of I, calponin; and J, OPN [scale bars: 100 μm; 50 μm; data are expressed as mean ± standard deviation (SD); statistical significance: * P < 0.05, ** P < 0.01; *** P < 0.001 and **** P < 0.0001].
4.4. Allicin Regulates Contractile Protein Expression and Reduces Aortic Dissection-Related Mortality
The VSMCs within the aortic media play a pivotal role in maintaining aortic wall integrity. Following exposure to pathological stimuli, VSMCs undergo phenotypic switching from contractile to synthetic states, a key pathogenic mechanism in AD. Immunofluorescence analysis revealed significant BAPN-induced downregulation of contractile markers α-SMA and SM22α in aortic sections, which was substantially attenuated by allicin co-treatment (Figure 2C and D). Western blotting demonstrated that PDGF-BB stimulation reduced α-SMA and SM22α expression in cultured VSMCs, effects that were reversed by allicin administration (Figure 2E - G). Concurrently, PDGF-BB treatment decreased calponin while increasing OPN expression compared to controls. Remarkably, allicin co-treatment restored calponin expression and normalized OPN levels to baseline values (Figure 2H - J).
4.5. Allicin Reduces Oxidative Stress Levels
Oxidative stress reflects an imbalance between pro-oxidant and antioxidant systems within the body, leading to impaired elastic fiber synthesis and contributing to AD. The PDGF-BB intervention group exhibited elevated levels of oxidative stress; however, allicin treatment increased SOD levels and reduced MDA levels (Figure 3A and B). Furthermore, the findings indicated that allicin reversed the green fluorescence enhancement caused by PDGF-BB, counteracting the ROS increase induced by PDGF-BB (Figure 3C). Thus, allicin overall mitigated the oxidative stress response induced by PDGF-BB.
Allicin can reduce levels of oxidative stress and inflammatory response. Three-week-old C57BL/6J male mice were treated with β-aminopropionitrile (BAPN) and allicin for 4 weeks. Mouse aortic smooth muscle cells were treated with platelet-derived growth factor-BB (PDGF-BB). A, superoxide dismutase (SOD); and B, malondialdehyde (MDA) levels in mouse aortic smooth muscle; C, color and quantification of reactive oxygen species (ROS, green) in aortic smooth muscle cells of mice in each group; D, interleukin-6 (IL-6) levels in aortic smooth muscle cells of mice in each group; E, tumor necrosis factor-alpha (TNF-α) levels in the smooth muscle cells of the aorta in each group of mice. Nuclei were stained with DAPI (blue); F, representative images of nuclear factor-kappa B (NF-κB, green) immunohistochemical staining in the aorta of each group and quantitative expression of NF-κB in aortic tissue; G, representative images of p38 (red) immunohistochemical staining in the aorta of each group and quantitative expression of p38 in aortic tissue [the data is expressed as mean ± standard deviation (SD); statistical significance: * P < 0.05, ** P < 0.01; *** P < 0.001 and **** P < 0.0001].
4.6. Allicin Reduces Inflammatory Reaction Levels
Inflammation plays a crucial role in the development and progression of AD (16). The IL-6 and TNF-α are the main pro-inflammatory cytokines, and their elevated levels are closely related to the progression of interlayer and AD-related complications (17). The levels of IL-6 and TNF-α were significantly increased in the cell group treated with PDGF-BB but returned to normal levels after administration of allicin (Figure 3D and E). The immunofluorescence staining results of animal experiments showed that 28 days after modeling, the density of NF-κB and p38 in the model group was significantly higher than that in the control group. However, allicin treatment partially reversed the expression of NF-κB and p38 (Figure 3F and G).
5. Discussion
The therapeutic potential of allicin against AD was investigated using a murine model. Given the limited availability of AD surgical specimens and challenges in obtaining appropriate normal controls (18), animal models remain essential for AD pathological studies (19). The established BAPN-induced AD model was found to recapitulate clinical AD manifestations, including aortic dilation, hematoma formation, and structural deterioration, confirming its utility for evaluating potential therapeutics.
The ECM abnormalities, recognized as hallmark pathological features of AD (20), primarily involve elastic/collagen fibers, with type I collagen being particularly abundant in aortic walls and critical for tensile strength (21). Previous investigations demonstrated that alpha-lipoic acid (ALA) attenuated BAPN-induced ECM degradation through suppression of FN and COL1A1 breakdown (22), correlating with reduced aortic rupture incidence. Similarly, the current findings demonstrate that allicin administration attenuates AD progression by mitigating ECM degradation through upregulated COL1A1 and FN expression.
Vascular remodeling in AD involves quantitative and phenotypic alterations of VSMCs within the aortic media. Under physiological conditions, VSMCs exhibit minimal proliferative and migratory activity (23). However, endothelial injury triggers pathological VSMC proliferation and migration toward the intimal layer, driving vascular remodeling (15). This process is characterized by a contractile-to-synthetic phenotypic transition, evidenced by reduced α-SMA and SM22α expression (24). In the present study, allicin treatment effectively reversed the downregulation of these contractile markers while suppressing PDGF-BB-induced VSMC proliferation and migration.
Oxidative stress has been identified as a central mediator of arterial wall remodeling, promoting AD pathogenesis through impaired elastin synthesis and elevated vascular shear stress (25). Clinical proteomic analyses revealed decreased SOD expression and increased MDA levels in AD patients' aortic tissues, indicating redox imbalance involvement (26). Elevated ROS levels were observed in model groups, which were normalized following allicin intervention, consistent with prior reports of allicin-mediated MDA reduction (27). Notably, oxidative stress exacerbates vascular damage via inflammatory cascade activation, as evidenced in both AD models and clinical cohorts (28, 29). Significantly elevated plasma TNF-α and IL-6 levels in AD patients, particularly IL-6's correlation with aneurysm rupture risk (17), were effectively counteracted by allicin treatment in this investigation.
The p38/MAPK signaling pathway, activated by external stimuli, induces NF-κB activation and oxidative stress elevation, subsequently triggering inflammatory responses (e.g., TNF-α upregulation) (30) and promoting VSMC phenotypic switching (marked by SM22α/α-SMA dysregulation) with concomitant proliferation/migration abnormalities. These pathological cascades ultimately result in ECM degradation (COL1A1, FN). Experimental data indicate that allicin administration significantly reduces inflammatory cytokine production, alleviates oxidative stress, prevents VSMC phenotypic modulation, restores ECM integrity, and ultimately decreases AD-associated mortality. These therapeutic effects may be mediated through inhibition of the p38/MAPK/NF-κB pathway. Future investigations employing p38 inhibitors and knockout models could elucidate allicin's oxidative stress modulation mechanisms.
In conclusion, our findings demonstrate that allicin effectively attenuates the progression of BAPN-induced AD in mice. The protective effects are mediated through the suppression of oxidative stress and inflammatory responses, preservation of the VSMC contractile phenotype, and inhibition of ECM degradation, likely via inhibition of the p38/MAPK and NF-κB signaling pathways. These results underscore the therapeutic potential of allicin in AD management. Nonetheless, this study has limitations. Future studies employing loss-of-function or gain-of-function strategies, along with clinical translational investigations, are warranted to fully elucidate its therapeutic value and molecular mechanisms.
![Allicin blunts extracellular matrix (ECM) degradation of the aorta and alleviates phenotype switch of vascular smooth muscle cell (VSMC): The 3-week-old C57BL/6J male mice were treated with β-aminopropionitrile (BAPN) and allicin for 4 weeks. Mouse aortic smooth muscle cells were treated with platelet-derived growth factor-BB (PDGF-BB). A, immunofluorescence staining and quantification of collagen type 1 (COL1A1, red) in aortas of each group; B, immunofluorescence staining and quantification of Fibronectin (red) in aortas from the Vehicle, PDGF-BB, and PDGF-BB + Allicin groups; C, immunofluorescence staining and quantification of smooth muscle protein 22-α (SM22α, red) in aortas of each group; D, immunofluorescence staining and quantification of alpha-smooth muscle actin (α-SMA, red) in aortas of each group. Nuclei were stained with DAPI (blue); E, representative western blot images of SM22α and α-SMA in each group during cell experiments. Quantification of F, SM22α; and G, α-SMA; H, representative western blot images of calponin and osteopontin (OPN) in each group. Quantification of I, calponin; and J, OPN [scale bars: 100 μm; 50 μm; data are expressed as mean ± standard deviation (SD); statistical significance: * P < 0.05, ** P < 0.01; *** P < 0.001 and **** P < 0.0001]. Allicin blunts extracellular matrix (ECM) degradation of the aorta and alleviates phenotype switch of vascular smooth muscle cell (VSMC): The 3-week-old C57BL/6J male mice were treated with β-aminopropionitrile (BAPN) and allicin for 4 weeks. Mouse aortic smooth muscle cells were treated with platelet-derived growth factor-BB (PDGF-BB). A, immunofluorescence staining and quantification of collagen type 1 (COL1A1, red) in aortas of each group; B, immunofluorescence staining and quantification of Fibronectin (red) in aortas from the Vehicle, PDGF-BB, and PDGF-BB + Allicin groups; C, immunofluorescence staining and quantification of smooth muscle protein 22-α (SM22α, red) in aortas of each group; D, immunofluorescence staining and quantification of alpha-smooth muscle actin (α-SMA, red) in aortas of each group. Nuclei were stained with DAPI (blue); E, representative western blot images of SM22α and α-SMA in each group during cell experiments. Quantification of F, SM22α; and G, α-SMA; H, representative western blot images of calponin and osteopontin (OPN) in each group. Quantification of I, calponin; and J, OPN [scale bars: 100 μm; 50 μm; data are expressed as mean ± standard deviation (SD); statistical significance: * P < 0.05, ** P < 0.01; *** P < 0.001 and **** P < 0.0001].](https://services.brieflands.com/cdn/serve/3170e/3b179faf301c6ff5366ef2b97314e863a300c602/jjnpp-20-4-165405-i002-preview.webp)
![Allicin can reduce levels of oxidative stress and inflammatory response. Three-week-old C57BL/6J male mice were treated with β-aminopropionitrile (BAPN) and allicin for 4 weeks. Mouse aortic smooth muscle cells were treated with platelet-derived growth factor-BB (PDGF-BB). A, superoxide dismutase (SOD); and B, malondialdehyde (MDA) levels in mouse aortic smooth muscle; C, color and quantification of reactive oxygen species (ROS, green) in aortic smooth muscle cells of mice in each group; D, interleukin-6 (IL-6) levels in aortic smooth muscle cells of mice in each group; E, tumor necrosis factor-alpha (TNF-α) levels in the smooth muscle cells of the aorta in each group of mice. Nuclei were stained with DAPI (blue); F, representative images of nuclear factor-kappa B (NF-κB, green) immunohistochemical staining in the aorta of each group and quantitative expression of NF-κB in aortic tissue; G, representative images of p38 (red) immunohistochemical staining in the aorta of each group and quantitative expression of p38 in aortic tissue [the data is expressed as mean ± standard deviation (SD); statistical significance: * P < 0.05, ** P < 0.01; *** P < 0.001 and **** P < 0.0001]. Allicin can reduce levels of oxidative stress and inflammatory response. Three-week-old C57BL/6J male mice were treated with β-aminopropionitrile (BAPN) and allicin for 4 weeks. Mouse aortic smooth muscle cells were treated with platelet-derived growth factor-BB (PDGF-BB). A, superoxide dismutase (SOD); and B, malondialdehyde (MDA) levels in mouse aortic smooth muscle; C, color and quantification of reactive oxygen species (ROS, green) in aortic smooth muscle cells of mice in each group; D, interleukin-6 (IL-6) levels in aortic smooth muscle cells of mice in each group; E, tumor necrosis factor-alpha (TNF-α) levels in the smooth muscle cells of the aorta in each group of mice. Nuclei were stained with DAPI (blue); F, representative images of nuclear factor-kappa B (NF-κB, green) immunohistochemical staining in the aorta of each group and quantitative expression of NF-κB in aortic tissue; G, representative images of p38 (red) immunohistochemical staining in the aorta of each group and quantitative expression of p38 in aortic tissue [the data is expressed as mean ± standard deviation (SD); statistical significance: * P < 0.05, ** P < 0.01; *** P < 0.001 and **** P < 0.0001].](https://services.brieflands.com/cdn/serve/3170e/5a3eaf15b16a6d29f551a88bff9cff4e2f5e1bab/jjnpp-20-4-165405-i003-preview.webp)