1. Background
Growing consumer demand for food safety and sustainability has driven interest in edible films from natural biopolymers like polysaccharides, proteins, and lipids (1). Chitosan (CH), derived from chitin deacetylation, is valued for its excellent film-forming and antibacterial properties in food packaging (2). Plant extracts can enhance the functional performance of these films by improving their antibacterial and antioxidant properties (3).
Scrophularia striata, a perennial herb containing bioactive compounds such as iridoids, flavonoids, and cinnamic acid, exhibits antioxidant, antimicrobial, anti-inflammatory, and immunomodulatory properties, as confirmed by several studies (4). Its hydroalcoholic extract shows protective effects against toxins via antioxidant mechanisms (5). Despite these properties, S. striata extracts in food packaging remain unexplored.
Trout fish (TF) is valued for its flavor and nutrition but is highly perishable. High water content, elevated pH, and nutrient richness accelerate spoilage through microbial growth, autolytic degradation, and lipid oxidation, exacerbated by high water activity (6). Research increasingly focuses on biopolymers and natural coatings as eco-friendly solutions for seafood preservation (6). Critical research gaps impede edible film applications, including insufficient understanding of food-coating interactions, limited interdisciplinary collaboration, inadequate biodegradability comparisons, and a lack of standardized evaluation methods (7). Limited mechanical and antioxidant testing, alongside a narrow focus on specific microbes rather than comprehensive microbial impacts, further restricts development. Addressing these gaps is crucial for advancing sustainable packaging (7).
2. Objectives
This study comprehensively evaluates CH films incorporating with S. striata extract (CH+SS) for trout fillet preservation, assessing chemical stability, microbial inhibition, morphological characteristics (SEM), molecular interactions (FTIR), and mechanical properties.
3. Methods
3.1. Materials
Fresh trout were purchased from local aquaculture farms. potato dextrose agar (PDA), cetrimide fucidin cephaloridine (CFC) agar, plate count agar (PCA), medium molecular weight CH (deacetylation degree 75 - 85%), glycerol, glutaraldehyde, butanol, dimethyl sulfoxide (DMSO), thiobarbituric acid (TBA), potassium iodide, sodium sulfate, magnesium oxide, boric acid, sulfuric acid, sodium thiosulfate, and 96% ethanol were obtained from Merck (Germany). Lyophilized strains of Salmonella typhimurium ATCC 14028, Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 25923, and Listeria monocytogenes ATCC 7644 were purchased from Darvash Company (Iran).
3.2. Fish Preparation and Sample Treatment
Ten aquacultured trout were sacrificed via spinal tap, transported on ice to the Food Microbiology Laboratory within one hour, cleaned, and filleted. Four fillets (approximately 120 g each) were obtained from each fish and randomly assigned to four treatment groups: (1) Control (untreated), (2) CH film coating, (3) S. striata extract dip (5% w/v solution), and (4) CH+SS (50 mg extract per g CH). All fillets were stored at 4°C for 12 days with periodic analysis.
3.3. Plant Extract Preparation
Scrophularia striata was authenticated (voucher specimen A2127801010A) by the Jundishapur Pharmacognosy Research Center, Ahvaz. Shade-dried aerial parts were ground to a fine powder (500 μm mesh size). Hydroalcoholic extraction was performed using 80% ethanol (1:10 plant: Solvent ratio) with continuous agitation for 24 hours at room temperature. The extract was filtered through Whatman No. 1 paper, concentrated using rotary evaporation at 45°C, and lyophilized. The dried extract was stored at 4°C until use (4).
3.4. Antimicrobial Activity Assessment
The antimicrobial activity of S. striata extract was evaluated against foodborne pathogens using disc diffusion and broth microdilution methods. For disc diffusion, Mueller-Hinton agar plates were inoculated with standardized bacterial suspensions (0.5 McFarland). Sterile filter paper discs (6 mm diameter) were impregnated with 100 μL of extract solutions (concentration range: 781.25 - 50,000 μg/mL in DMSO) and placed on inoculated agar. Plates were incubated at 37°C for 24 hours, and inhibition zones were measured in millimeters. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined using the broth microdilution method in 96-well plates according to CLSI guidelines. Extract concentrations/extract-containing coatings were prepared in concentrations from 12,500, 9,375, 6,250, 4,687.5, 3,125, 1,562.5, 781.25, 390.62, and 195.31 µg/mL. 20 µL of each concentration was added to microplate wells containing 160 µL BHI broth and 20 µL bacterial suspension.
3.5. Film Preparation and Characterization
The CH films were prepared by dissolving 2% (w/v) CH in a 1% acetic acid solution with continuous stirring at 60°C for 2 hours. Glycerol (0.3 mL/g CH) was added as a plasticizer, and S. striata extract (50 mg/g CH) was incorporated for active films. The film-forming solution was cast onto Petri dishes and dried in a controlled environment chamber at 25°C and 50% relative humidity for 24 hours. All instruments and surfaces were sterilized with 70% ethanol prior to use (8). Six film formulations were prepared:
1. CHPGE: High MW CH + PVA + glycerol + extract.
2. CHPG: High MW CH + PVA + glycerol.
3. CHG: High MW CH + glycerol.
4. CLPGE: Low MW CH + PVA + glycerol + extract.
5. CLPG: Low MW CH + PVA + glycerol.
6. CLG: Low MW CH + glycerol.
Film thickness was measured at 10 random positions using a digital micrometer (9). Mechanical properties (tensile strength and elongation at break) were determined using a texture analyzer according to ASTM D882-12. Film morphology was examined by scanning electron microscopy, and molecular interactions were analyzed by FTIR spectroscopy (9).
3.6. Analytical Methods
3.6.1. pH Measurement
A fish homogenate (10 g in 100 mL distilled water) was prepared using a stomacher, and the pH was measured with a calibrated pH meter (3).
3.6.2. TBARS Analysis
Lipid oxidation was determined using the TBA reactive substances method. A sample (0.2 g fat) was dissolved in butanol, reacted with TBA reagent at 95°C for 2 hours, and the absorbance was measured at 532 nm (10).
3.6.3. TVB-N Determination
Total volatile basic nitrogen was measured using the micro-Kjeldahl distillation method. A sample (10 g) was alkalinized with magnesium oxide and distilled, with the distillate collected in a boric acid solution and titrated with sulfuric acid (11).
3.6.4. Peroxide Value
Lipid hydroperoxides were determined by the iodometric titration method according to AOCS Cd 8-53 (12).
3.7. Microbiological Analysis
Fish samples (10 g) were homogenized with 90 mL sterile peptone water using a stomacher. Serial decimal dilutions were prepared and plated on appropriate media: The PCA for total viable count (37°C/48 h), PCA for psychrotrophic count (7°C/10 days), CFC for Pseudomonas count (20°C/48 h), and PDA for yeast and mold count (25°C/5 - 7 days). Results were expressed as log CFU/g (13).
3.8. Sensory Evaluation
Trained panelists (n = 15) from the University staff evaluated the raw fillets for color, odor, and texture attributes using a 5-point hedonic scale (1 = excellent, 5 = unacceptable) (3). Evaluation was conducted under controlled lighting conditions with randomized sample presentation. Panelists provided informed consent prior to participation.
3.9. Statistical Analysis
All experiments were conducted in triplicate. Data were analyzed using SPSS version 21.0. One-way ANOVA with Tukey's post-hoc test was used for multiple comparisons. Repeated measures ANOVA was used for time-course data. Significance was determined at P < 0.05. In Figures 1 and 2, shared lowercase and uppercase letters denote no significant difference (P > 0.05) between treatments and storage days within a treatment, respectively.
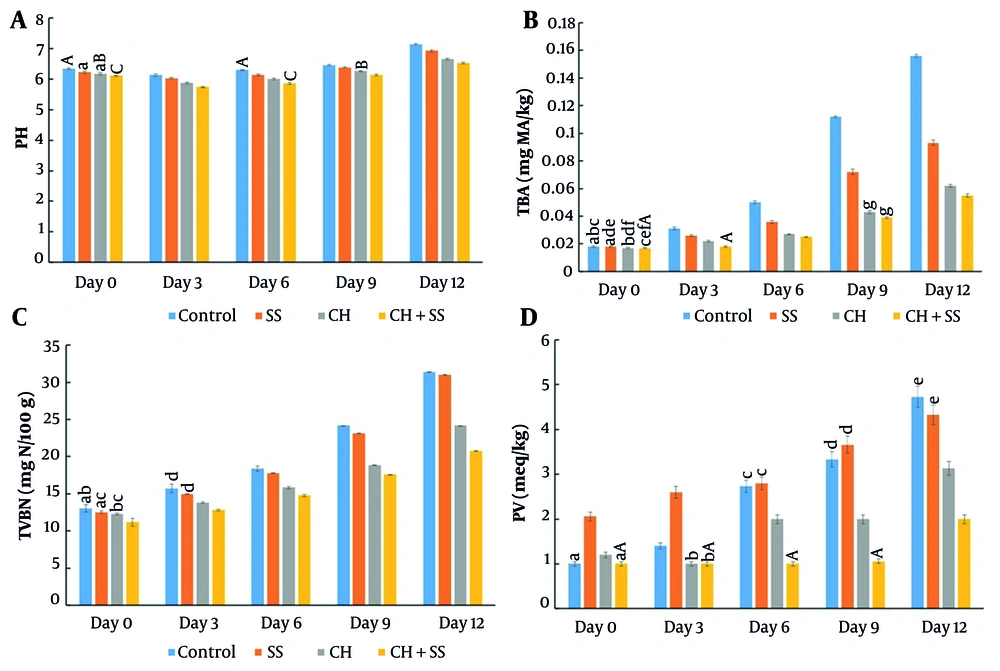
Changes in chemical quality parameters of trout fillets during 12-day storage at 4°C: A, pH; B, TBARS; C, TVB-N; and D, peroxide values (lowercase letters indicate no significant difference between treatments; capital letters indicate no significant difference between storage days within the same treatment; values are expressed as mean ± SD, n = 3; P > 0.05).
Microbial dynamics in trout fillets during 12-day storage at 4°C: A, total viable count (TVC); B, psychrotrophic bacteria count (PTC); C, Pseudomonas count (PSC); and D, yeast and mold count [data represent mean ± SD for control, chitosan (CH), S. striata extract, and CH film incorporating with S. striata extract (CH+SS) treatment groups (log CFU/g). Lowercase letters indicate no significant difference between treatments; capital letters indicate no significant difference between storage days within the same treatment; P > 0.05].
4. Results
4.1. Film Physical Properties
The thickness measurements revealed significant differences between film formulations. Low molecular weight CH films (CLPGE, CLPG, CLG) averaged 0.10 ± 0.01 mm, while high molecular weight variants (CHPGE, CHPG, CHG) measured 0.11 ± 0.01 mm. Incorporation of S. striata extract increased film thickness by approximately 15% in high molecular weight films (P < 0.05) (12, 13).
4.2. Mechanical Performance
Mechanical testing demonstrated formulation-dependent properties (Table 1). CHG films showed the lowest tensile strength (18.3 ± 1.5 MPa), while CLPG films exhibited optimal mechanical properties with a tensile strength of 28.4 ± 1.2 MPa and elongation at break of 42.3 ± 3.1%. Based on the results of mechanical testing (Table 1), the CLPG formulation was selected as the optimal base film due to its superior tensile strength and flexibility. This formulation was then functionalized by incorporating S. striata extract (creating the CH+SS treatment group) for application on trout fillets to assess preservative efficacy.
| Films | TS (MPa) | Elong Aft Break (%) | Module (MPa) |
|---|---|---|---|
| CHPGE | 2.73 ± 0.25 | 7.39 ± 1.21 | 7.89 ± 0.50 |
| CHPG | 4.08 ± 1.44 | 7.55 ± 3.31 | 11.16 ± 0.23 |
| CHG | 1.18 ± 0.06 | 3.68 ± 0.06 | 6.38 ± 0.49 |
| CLPGE | 8.54 ± 2.04 | 36.27 ± 10.27 | 20.34 ± 2.58 |
| CLPG | 14.92 ± 0.10 | 40.07 ± 7.88 | 24.12 ± 1.73 |
| CLG | 2.53 ± 0.28 | 11.35 ± 0.89 | 6.54 ± 1.55 |
a Values are expressed as mean ± SD.
4.3. Morphological Characteristics
SEM analysis demonstrated distinct surface morphological differences (Figures 3A and B). Pure CH films exhibited smooth, homogeneous surfaces, while S. striata extract-incorporated films showed increased surface roughness with visible particulate aggregation and micro-pores. The composite films displayed a dense sub-layer structure, suggesting potential barrier property enhancement.
4.4. Molecular Interactions
FTIR spectral analysis revealed characteristic peaks (Figure 3C): 3278 cm-1 (O-H/N-H stretching), 2800 - 2920 cm-1 (C-H stretching), 1620 - 1720 cm-1 (C=O stretching), 1557 cm-1 (N-H bending), 1409 cm-1 (O-H bending), and 1026 cm-1 (C-O/C-N stretching). The CH+SS spectrum showed minimal peak shifts, notably a carbonyl group shift to 1680 - 1730 cm-1, indicating physical interactions through hydrogen bonding.
4.5. Antimicrobial Efficacy
Antimicrobial assessment revealed concentration-dependent activity (Tables 2 - 4). S. striata extract (50,000 μg/mL) produced inhibition zones of 9.87 ± 1.7 mm (L. monocytogenes), 10.8 ± 0.21 mm (S. typhimurium), 13.56 ± 0.33 mm (P. aeruginosa), and 12.83 ± 0.24 mm (S. aureus). The MIC values ranged from 6250 - 9375 μg/mL with an MBC of 12500 μg/mL for all pathogens. The CH+SS films showed enhanced activity, particularly against S. aureus (MIC = 195.31 μg/mL) and P. aeruginosa (MIC = 4687.5 μg/mL).
| Concentration (µg/mL) | Listeria monocytogenes | Salmonella typhimurium | Pseudomonas aeruginosa | Staphylococcus aureus |
|---|---|---|---|---|
| 50000 | ND | 17.43 ± 0.23 | 19.33 ± 0.32 | 25.33 ± 0.58 |
| 25000 | ND | 14.33 ± 0.49 | 14.73 ± 0.06 | 21.50 ± 0.50 |
| 12500 | ND | 13.80 ± 0.30 | 11.60 ± 0.30 | 15.50 ± 0.25 |
| 6250 | ND | 11.40 ± 0.53 | ND | 13.66 ± 0.58 |
| 3125 | ND | 10.40 ± 0.26 | ND | 13.00 ± 0.00 |
| 1562.5 | ND | ND | ND | 12.26 ± 0.15 |
| 781.25 | ND | ND | ND | 11.63 ± 0.31 |
Abbreviation: ND, not detectable.
a Values are expressed as mean ± SD.
b Larger inhibition zone diameters indicate greater antimicrobial activity.
| Foodborne Pathogens | Listeria monocytogenes | Salmonella typhimurium | Pseudomonas aeruginosa | Staphylococcus aureus |
|---|---|---|---|---|
| Inhibition zone | 9.87 ± 1.27 | 10.8 ± 0.21 | 13.56 ± 0.33 | 12.83 ± 0.24 |
a Values are expressed as mean ± SD.
| Food Pathogens | Listeria monocytogenes (µg/mL) | Salmonella typhimurium (µg/mL) | Pseudomonas aeruginosa (µg/mL) | Staphylococcus aureus (µg/mL) |
|---|---|---|---|---|
| Scrophulariastriata Extract | ||||
| MIC | 6250 | 9375 | 6250 | 6250 |
| MBC | 12500 | 12500 | 12500 | 12500 |
| CH+SS | ||||
| MIC | 9375 | 9375 | 4687.5 | 195.31 |
| MBC | 12500 | 12500 | 6250 | 1562.5 |
Abbreviations: MIC, minimum inhibitory concentration; MBC, minimum bactericidal concentration; CH+SS, CH film incorporating with S. striata extract.
4.6. Chemical Quality Parameters
Storage studies revealed significant treatment effects on quality parameters (Figure 1): (A) pH values showed an initial decrease followed by a gradual increase, with CH+SS maintaining the most stable profile (6.3 ± 0.1 at day 12); (B) TBARS values increased minimally in CH+SS (0.017 to 0.056 mg MDA/kg); (C) TVB-N remained within acceptable limits in CH (24.18 ± 0.01 mg/100g) and CH+SS (20.75 ± 0.02 mg/100g); (D) peroxide values showed the best oxidative stability in CH+SS films.
4.7. Microbial Dynamics
Microbial analysis demonstrated preservation efficacy (Figure 2): (A) TVC maintained at 4.89 ± 0.02 log CFU/g in CH+SS vs 7.53 ± 0.03 log CFU/g in control at day 12; (B) Pseudomonas counts were undetectable in CH+SS throughout storage; (C) psychrotrophic counts were lowest in CH+SS (5.65 ± 0.08 log CFU/g at day 12); (D) mold/yeast counts exceeded acceptable limits in all groups by day 6, though the slowest growth was in CH+SS.
4.8. Sensory Evaluation
Sensory assessment revealed maintained acceptability in treated samples (Figure 4). The CH and CH+SS maintained scores > 3 throughout storage, while the control became unacceptable (score < 2) by day 6. Significant differences were observed in color, odor, and texture attributes (P < 0.05), with CH+SS showing optimal sensory preservation.
5. Discussion
The findings demonstrate significant enhancement of trout fillet preservation through CH+SS. The improved functional properties observed in CH+SS films align with previous studies investigating bioactive composite films (13, 14). The mechanical property enhancement in low molecular weight CH formulations contradicts some literature (13) but may be attributed to better integration of plasticizers and extract components in less viscous solutions. The use of synthetic but biodegradable PVA was a necessary compromise to achieve the mechanical integrity required for practical application, highlighting a common challenge in developing fully bio-based films with competitive performance.
The antimicrobial efficacy observed, particularly against gram-negative pathogens, suggests synergistic action between CH's membrane disruption properties and the S. striata extract's bioactive compounds. The exceptional performance against P. aeruginosa (undetectable counts throughout storage) represents a significant finding, as Pseudomonas species are primary spoilage organisms in refrigerated fish products (15, 16). The reduced efficacy against mold and yeast species indicates potential limitations for long-term storage applications requiring broad-spectrum antifungal activity.
Chemical parameter stabilization, particularly TVB-N maintenance within acceptable limits, demonstrates the films' effectiveness in inhibiting proteolytic spoilage mechanisms. The TBARS and peroxide value results indicate significant antioxidant activity, likely attributable to phenolic compounds in S. striata extract, preventing lipid oxidation (4, 17). The pH stability in treated samples suggests inhibition of both microbial metabolism and autolytic enzyme activity.
Sensory preservation correlates strongly with microbial and chemical results, supporting the practical applicability of CH+SS coatings. The maintained sensory acceptability throughout the 12-day storage period extends shelf life by 2 - 3 days compared to conventional preservation methods (3, 18). The correlation between microbial load and sensory deterioration underscores the importance of antimicrobial efficacy in shelf-life extension. The physical interaction mechanism suggested by FTIR analysis indicates potential for an improved safety profile compared to chemical modification approaches. However, the observed extract aggregation in SEM images suggests opportunities for formulation optimization through improved homogenization techniques or surfactant addition.
This study establishes the foundation for utilizing S. striata extract in food packaging applications, particularly for seafood preservation. Future research should focus on the optimization of extract concentration, investigation of specific active compounds, and the use of larger sample sizes to strengthen statistical power. Additionally, assessment of sensory impact at higher concentrations, scale-up studies, and economic feasibility analysis will be crucial for facilitating the commercial application of this promising preservation technology.
5.1. Conclusions
This study demonstrates that CH+SS significantly enhanced the preservation of trout fillets during refrigerated storage. The CH+SS composite films effectively maintained chemical stability by controlling pH, lipid oxidation, and protein degradation while demonstrating exceptional antimicrobial activity, particularly against Pseudomonas species. Sensory evaluation confirmed that these films maintained acceptable quality attributes throughout the 12-day storage period. The main limitation of this study is the low effectiveness on mold and yeast growth. It is suggested that an anti-mold-yeast preservative be used in membrane preparation. These findings establish S. striata extract as a promising natural bioactive agent for food packaging applications. Future research should focus on optimizing extract concentration, developing nano-formulations, and exploring Pickering emulsion systems to further enhance the preservation efficacy and practical applicability of this innovative approach, in addition to conducting consumer acceptability testing of cooked fillets to fully assess the impact on sensory perception and market potential.
![Microbial dynamics in trout fillets during 12-day storage at 4°C: A, total viable count (TVC); B, psychrotrophic bacteria count (PTC); C, <i>Pseudomonas</i> count (PSC); and D, yeast and mold count [data represent mean ± SD for control, chitosan (CH), <i>S. striata</i> extract, and CH film incorporating with <i>S. striata</i> extract (CH+SS) treatment groups (log CFU/g). Lowercase letters indicate no significant difference between treatments; capital letters indicate no significant difference between storage days within the same treatment; P > 0.05]. Microbial dynamics in trout fillets during 12-day storage at 4°C: A, total viable count (TVC); B, psychrotrophic bacteria count (PTC); C, <i>Pseudomonas</i> count (PSC); and D, yeast and mold count [data represent mean ± SD for control, chitosan (CH), <i>S. striata</i> extract, and CH film incorporating with <i>S. striata</i> extract (CH+SS) treatment groups (log CFU/g). Lowercase letters indicate no significant difference between treatments; capital letters indicate no significant difference between storage days within the same treatment; P > 0.05].](https://services.brieflands.com/cdn/serve/3170d/9e35b3d39d4d583e1f14dca61bac07be9382de57/jjnpp-20-4-165986-i002-preview.webp)
![Surface morphology of chitosan films: A, pure chitosan (CH); and B, CH film incorporating with <i>Scrophularia striata</i> extract [CH+SS; FTIR spectra of; C.a, CH film and C.b, CH+SS composite film]. Surface morphology of chitosan films: A, pure chitosan (CH); and B, CH film incorporating with <i>Scrophularia striata</i> extract [CH+SS; FTIR spectra of; C.a, CH film and C.b, CH+SS composite film].](https://services.brieflands.com/cdn/serve/3170d/c2cc6512afa38a7873535ff3922042b9e717ba30/jjnpp-20-4-165986-i003-preview.webp)
![Sensory evaluation of trout fillets during 12-day storage at 4°C: A, color scores; B, odor scores; and C, texture scores [data represent mean ± SD of sensory for control, chitosan (CH), <i>S. striata</i> extract, and CH film incorporating with <i>S. striata</i> extract (CH+SS) treatment groups]. Sensory evaluation of trout fillets during 12-day storage at 4°C: A, color scores; B, odor scores; and C, texture scores [data represent mean ± SD of sensory for control, chitosan (CH), <i>S. striata</i> extract, and CH film incorporating with <i>S. striata</i> extract (CH+SS) treatment groups].](https://services.brieflands.com/cdn/serve/3170d/bc9addbefb60fc93cab1dabf97d9fc55ab714eb9/jjnpp-20-4-165986-i004-preview.webp)