1. Background
Methamphetamine is a potent psychostimulant that increases the release and inhibits the reuptake of monoamine neurotransmitters, including dopamine, norepinephrine, and serotonin (1). It is most commonly consumed by smoking or nasal insufflation, though oral and intravenous routes are also used. The clinical effects of methamphetamine include heightened energy and alertness, euphoria, activation of the sympathetic nervous system, decreased need for sleep, weight loss, and dry mouth, which often leads to dental decay. Chronic use is associated with a range of psychiatric and cognitive impairments, such as irritability, anxiety, aggression, paranoia, hallucinations, executive dysfunction, and memory deficits. Furthermore, methamphetamine use may exacerbate preexisting psychiatric conditions (2).
According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), methamphetamine-related psychiatric diagnoses are classified under stimulant use disorders (3). Globally, amphetamine-type stimulants (ATS) — particularly methamphetamine — constitute one of the fastest-growing classes of illicit substances (4, 5). The use of ATS has increased substantially in Asia and Oceania, making them the second most commonly used drug class worldwide (6).
Iran, located along a major drug trafficking corridor from Afghanistan and Pakistan to Europe, experiences a high burden of substance use. Estimates indicate that between 2 and 4 million Iranians misuse substances, with approximately 1.2 million meeting diagnostic criteria for drug dependence (7). While opium and heroin have historically been the most prevalent substances, recent trends show a shift toward crystal heroin and methamphetamine (8, 9). Surveys report that amphetamine-type stimulant use in Iran ranges from 3.6% to 15%, with an average age of initiation around 21 years (10-13).
Methamphetamine use is associated with markedly elevated mortality. In a retrospective study involving 1,254 hospitalized methamphetamine users, the five-year mortality rate was 5%, with women experiencing a 26-fold and men a 6-fold increase in mortality risk compared to the general population (14). Additionally, suicide attempts are more prevalent among methamphetamine users than among non-users (15).
Despite the growing prevalence of methamphetamine use disorder (MUD), there is currently no US Food and Drug Administration (FDA)-approved pharmacotherapy. A 2020 systematic review encompassing 43 studies with 4,065 patients and 23 medications identified the most promising outcomes for stimulant agonists (such as dextroamphetamine and methylphenidate), naltrexone, and topiramate. Antidepressants, including bupropion and mirtazapine, demonstrated mixed and generally modest effects (16). It is notable that most mirtazapine studies have focused on specific subpopulations, such as men who have sex with men, highlighting the need for broader research.
Preclinical studies provide evidence that mirtazapine may attenuate methamphetamine-induced behaviors. For example, Nabavi et al. demonstrated that mirtazapine blocked methamphetamine-induced conditioned place preference in mice in a dose- and duration-dependent manner (17). In human clinical trials, mirtazapine has shown potential in reducing craving and improving psychiatric symptoms in stimulant users (18).
Mirtazapine is a tetracyclic antidepressant approved by the FDA, recognized for its rapid onset of action (typically within two weeks) and side effects including sedation and weight gain (19, 20). It functions as a noradrenergic and specific serotonergic antidepressant (NaSSA), enhancing the release of norepinephrine, serotonin, and dopamine in mesocorticolimbic pathways that are implicated in reward, craving, and drug-seeking behavior (21-23). This neurochemical profile offers a plausible mechanism for reducing methamphetamine craving and withdrawal symptoms (15).
2. Objectives
Given the limited pharmacological options for MUD and the promising preliminary evidence supporting mirtazapine, the present study aimed to evaluate its efficacy in reducing craving and depressive symptoms among patients with amphetamine and methamphetamine use disorder (AMD) attending addiction treatment centers in Ahvaz, Iran.
3. Methods
This study was a double-blind, placebo-controlled randomized clinical trial designed to evaluate the effect of mirtazapine on reducing amphetamine and methamphetamine use among patients attending addiction treatment clinics at Golestan, Imam Khomeini, Sina, and Taleghani hospitals, as well as private centers in Ahvaz, Iran. A total of 84 patients diagnosed with amphetamine-type stimulant use disorder according to the DSM-5 criteria were recruited by psychiatric specialists. Participants were randomly assigned to either the intervention group (mirtazapine) or the control group (placebo), with 42 patients in each arm. Randomization was conducted using block randomization with concealed allocation, and the random sequence was generated by an independent statistician.
The inclusion criteria were: Diagnosis of stimulant use disorder based on the Structured Clinical Interview for DSM-5 (SCID-5); willingness to reduce or quit methamphetamine use; at least one positive urine test for methamphetamine during screening; age between 18 and 60 years; and normal baseline laboratory tests, including complete blood count (CBC), glucose, creatinine, blood urea nitrogen (BUN), liver enzymes, and electrolytes. All participants provided written informed consent prior to enrollment. Exclusion criteria included: Diagnosis of psychotic disorders, major depressive disorder, or bipolar disorder; hypersensitivity to mirtazapine; recent use of antidepressants [except selective serotonin reuptake inhibitors (SSRIs)]; severe hepatic or renal impairment; legal constraints (such as risk of incarceration); or any condition deemed unsafe by the investigator.
After enrollment, the intervention group received mirtazapine tablets, starting at 15 mg daily and increased to 30 mg after one week. The control group received a matched placebo identical in appearance, taste, and smell. Both medications were administered orally at bedtime for 12 weeks. Participants, clinicians, and outcome assessors remained blinded throughout the study.
3.1. Placebo Preparation and Blinding Integrity
Placebo tablets were manufactured by the School of Pharmacy at Jundishapur University of Medical Sciences and coded identically to mirtazapine tablets. Packaging and distribution were performed under blinded conditions to ensure allocation concealment.
3.2. Assessments and Follow-up
Physical examinations and vital signs were recorded at baseline and every two weeks. Craving was assessed biweekly using a validated 20-item craving questionnaire (Cronbach’s α = 0.89), scored on a 6-point Likert scale. Depression was measured monthly via the Beck Depression Inventory (BDI), a 21-item scale validated in Iranian populations. Addiction severity was evaluated biweekly using the fifth edition of the Addiction Severity Index (ASI), covering medical, occupational, legal, family, and psychological domains. Urine drug tests for amphetamines and methamphetamines were conducted biweekly.
3.3. Medication Adherence and Protocol Deviations
Adherence was monitored through pill counts and self-reports. The overall adherence rate was 92.8%. Seven participants discontinued due to mild side effects or missed visits. All discontinuations were documented, and follow-up data were collected when possible.
3.4. Safety Monitoring
Discontinuation criteria included serious adverse events, patient request, or investigator judgment. Mild side effects — such as dry mouth, drowsiness, and appetite changes — were recorded and managed conservatively.
3.5. Sample Size Calculation
Based on previous studies and a longitudinal design, a sample size of 42 per group was calculated using G*Power software. The assumptions included a medium effect size (Cohen’s d = 0.5), α = 0.05, and power = 0.95.
3.6. Statistical Analysis
Descriptive statistics were used for demographic data. Normality was assessed using the Kolmogorov-Smirnov test. Repeated measures analysis of variance (ANOVA) with Greenhouse-Geisser correction was applied to analyze changes in craving and depression scores over time. Generalized estimating equations (GEEs) were used for longitudinal trends. All analyses were performed using SPSS version 24 (IBM Corp., Armonk, NY, USA), with statistical significance set at P < 0.05.
3.7. Trial Registration
This study was registered in the Iranian Registry of Clinical Trials (IRCT20230606058397N1).
4. Results
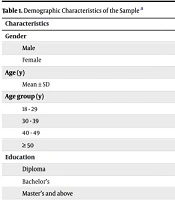
The clinical trial enrolled 84 participants, including 57 men (67.9%) and 27 women (32.1%). The mean age was 34.7 ± 8.9 years, with participants ranging from 18 to 65 years of age. The most common age group was 30 - 39 years, representing 22.5% of the sample. Regarding educational attainment, 21 participants (25%) held a diploma, 38 (45%) had a bachelor’s degree, and 25 (30%) possessed a master’s degree or higher. In terms of marital status, 50 participants (59.5%) were married, while 34 (40.5%) were single (Table 1).
| Characteristics | Values |
|---|---|
| Gender | |
| Male | 57 (67.9) |
| Female | 27 (32.1) |
| Age (y) | |
| Mean ± SD | 34.7 ± 8.9 |
| Age group (y) | |
| 18 - 29 | 25 (29.8) |
| 30 - 39 | 19 (22.6) |
| 40 - 49 | 22 (26.2) |
| ≥ 50 | 18 (21.4) |
| Education | |
| Diploma | 21 (25.0) |
| Bachelor’s | 38 (45.2) |
| Master’s and above | 25 (29.8) |
| Marital status | |
| Married | 50 (59.5) |
| Single | 34 (40.5) |
a Values are expressed as No. (%) unless indicated.
Mental health status was assessed at baseline using a validated screening tool, specifically the General Health Questionnaire-28 (GHQ-28). The mean mental health score among the 84 clinical trial participants was 23.5 ± 6.7, which generally indicates moderate psychological well-being. However, approximately 30% of participants (n = 25) scored above the clinical cut-off threshold, suggesting the presence of potential psychological disorders within this subgroup (Table 2).
| Mental Health Status | Male (N = 57) | Female (N = 27) | Total (N = 84) |
|---|---|---|---|
| Healthy | 40 | 19 | 59 (70.2) |
| Psychological issue | 17 | 8 | 25 (29.8) |
a Values are expressed as No. (%).
A chi-square test was conducted to examine the relationship between gender and mental health status. The results indicated no statistically significant association (χ2 = 0.24, P = 0.62), suggesting that psychological distress was not dependent on gender in this sample. Job satisfaction was evaluated using a standardized questionnaire, with scores ranging from 1 to 5. The mean job satisfaction score was 3.8 ± 0.9, reflecting moderate overall satisfaction among participants. Additionally, 40% of participants (n = 34) reported high job satisfaction (score ≥ 4) (Table 3).
| Age Group (y) | Mean Satisfaction ± SD |
|---|---|
| 18 - 29 | 3.5 ± 0.8 |
| 30 - 39 | 4.0 ± 0.9 |
| 40 - 49 | 3.9 ± 0.7 |
| ≥ 50 | 3.7 ± 1.0 |
| Total | 3.8 ± 0.9 |
The ANOVA revealed a significant difference in job satisfaction across age groups (F = 3.45, p = 0.018), with the 30 - 39 age group reporting the highest levels of satisfaction. Among the 84 patients in the clinical trial subset, 57% had a history of admission to rehabilitation camps, while 27% did not. All patients presented with active psychiatric problems; none were free of active psychiatric issues. Regarding psychiatric history, 41% of participants reported a prior psychiatric disorder, whereas 43% did not. Additionally, 44% had a prior medical history, while 40% reported no such history. For medication history, 38% had previously used medications, while 46% had not. A family history of addiction was present in 50% of patients and absent in 34%. Furthermore, 51% of participants had legal and social problems — such as family conflicts, imprisonment, desertion, or involvement in drug trafficking — while 33% did not report such issues (Table 4).
| Variables | No. (%) |
|---|---|
| History of camp admission | |
| Yes | 57 (67.9) |
| No | 27 (32.1) |
| Current mild psychiatric problem | |
| Yes | 84 (100) |
| No | 0 (0) |
| Previous psychiatric disorder | |
| Yes | 41 (48.8) |
| No | 43 (51.2) |
| Previous medical history | |
| Yes | 44 (52.4) |
| No | 40 (47.6) |
| History of medication use | |
| Yes | 38 (45.2) |
| No | 46 (54.8) |
| Family history of addiction | |
| Yes | 50 (59.5) |
| No | 34 (40.5) |
| Personal legal/social issues | |
| Family conflict, imprisonment, desertion, and drug sales | 51 (47.1) |
| No | 33 (39.3) |
Urine amphetamine tests conducted during three treatment sessions showed that, in the first session, all patients in both the intervention and control groups tested positive. In the second and third sessions, the proportion of positive tests in the intervention group decreased to 97.6% and 95.1%, respectively, while rates in the control group remained nearly unchanged. Although this difference approached statistical significance (P ≈ 0.05), the intervention group exhibited a clear decreasing trend.
Mean daily amphetamine consumption and daily cost also declined in the intervention group across the three sessions, whereas no significant changes were observed in the control group.
Regarding side effects, dry mouth and thirst were slightly more prevalent in the intervention group (P < 0.05); however, the incidence of other side effects — including drowsiness, weight gain, anxiety, and abdominal pain — did not differ significantly between the groups. Overall, mirtazapine was well tolerated, with side effects being mild and transient (Table 5).
| Variables | Intervention Group | Control Group | P-Value |
|---|---|---|---|
| Positive test rate session 3 | 95.1 | 97.6 | 0.05 |
| Mean daily use (g) | 0.17 ± 0.31 | 0.21 ± 0.20 | 0.08 |
| Daily cost (toman) | 26307.5 ± 316.36 | 32497.5 ± 254.24 | 0.07 |
| Other substances use (session 3) | 16.6 | 33.3 | 0.03 |
| Dry mouth | 0 | 7.1 | 0.02 |
| Thirst | 11.9 | 0 | 0.02 |
| Other side effects | < 5 in both groups | < 5 in both groups | NS |
Abbreviation: NS, not significant.
a Values are expressed as percentage or mean ± SD.
During sessions 4 to 6, the proportion of positive amphetamine urine tests in the intervention group decreased from 76.2% to 47.6%, whereas the control group remained consistently positive. The difference between groups in session 5 was statistically significant (P = 0.007). Daily amphetamine consumption and associated costs continued to decline in the intervention group, although most differences were not statistically significant. Use of other substances was higher in the control group, with a significant difference observed in session 5 (P = 0.04). Common side effects, including drowsiness and dry mouth, did not differ significantly between the groups (Table 6).
| Test Result | Session 4 (Intervention) | Session 4 (Control) | Session 5 (Intervention) |
|---|---|---|---|
| Positive | 76.2 | 95 | 64.3 |
| Negative | 23.8 | 4.8 | 35.7 |
| P-value | 0.35 (NS) | 0.007 | 0.43 (NS) |
| Mean daily use (g) | 0.21 ± 0.14 | 0.14 ± 0.20 | 0.08 ± 0.10 |
| Other substances | 16.7 | 33.3 | 14.3 |
| P-value | 0.15 (NS) | 0.04 | 0.02 |
Abbreviation: NS, not significant.
a Values are expressed as percentage or mean ± SD.
It was observed that the mean craving score in the intervention group steadily decreased over the course of the treatment sessions. In the first session, the mean craving score was 84.94 in the control group and 83.83 in the intervention group. By the sixth session, these values had decreased to 80.25 and 70.77, respectively. This downward trend in the intervention group suggests a positive effect of mirtazapine on reducing the mental urge and craving for stimulant substances (Table 7).
| Sessions | Intervention Group | Control Group |
|---|---|---|
| 1 | 83.83 ± 17.48 | 84.94 ± 9.70 |
| 2 | 82.99 ± 11.27 | 83.11 ± 19.73 |
| 3 | 78.30 ± 12.32 | 83.52 ± 12.61 |
| 4 | 72.05 ± 13.22 | 82.30 ± 16.74 |
| 5 | 68.64 ± 12.57 | 81.25 ± 18.39 |
| 6 | 70.77 ± 12.75 | 80.25 ± 12.57 |
a Values are expressed as mean ± SD.
To statistically evaluate changes in craving scores over time and between groups, repeated measures ANOVA with Greenhouse-Geisser correction was employed. The results indicated a significant decrease in craving scores over time (P < 0.001), as well as a significant difference between the two groups (P = 0.04). These findings demonstrate a significant therapeutic effect of mirtazapine in reducing amphetamine and methamphetamine craving (Table 8).
| Variables | Sum of Squares | Degrees of Freedom | Mean Square | F Statistic | P-Value |
|---|---|---|---|---|---|
| Craving (time) | 21729.30 | 2.42 | 8974.74 | 16.45 | < 0.001 |
| Group | 42.83 | 1 | 42.83 | 2.54 | 0.04 |
5. Discussion
The present study aimed to evaluate the effectiveness of mirtazapine in reducing dependence and craving among patients with methamphetamine and amphetamine use disorder referred to addiction treatment clinics at several centers in Ahvaz. The findings demonstrated that mirtazapine use in the intervention group led to a significant reduction in both craving and depression scores compared to the control group. Specifically, the mean depression score at the first session was 46.44 in the control group and 44.44 in the intervention group, which decreased to 42.85 and 34.78, respectively, by the sixth session, indicating a positive therapeutic effect (P < 0.05). In addition, repeated measures analysis with Greenhouse-Geisser correction revealed a significant difference in craving scores between the intervention and control groups, with notably lower scores observed in the intervention group (P < 0.05).
Importantly, side effects such as drowsiness, dry mouth, and appetite changes did not differ significantly between groups, suggesting good tolerability of the medication. Given the lack of approved pharmacological treatments for MUD, identifying an effective medication is crucial for managing this condition. The practical and inclusive design of our study allowed for observation of real-world effects, indicating that mirtazapine could be rapidly implemented in clinical practice and significantly improve treatment coverage for MUD (24).
Previous studies have also indicated the potential efficacy of mirtazapine. For example, a 12-week randomized controlled trial by Coffin et al., entitled "Mirtazapine to Reduce Methamphetamine Use", found that the mirtazapine group had a lower rate of positive urine tests for methamphetamine compared to placebo (hazard ratio 0.57; 95% CI, 0.35 - 0.93; P = 0.02) (25). Another systematic review and meta-analysis reported that mirtazapine likely leads to a modest reduction in methamphetamine use but does not significantly affect depressive symptoms (hazard ratio 0.81; 95% CI, 0.63 - 1.03; moderate certainty) (26).
Mechanistically, mirtazapine acts as a fourth-generation antidepressant with monoaminergic agonist-antagonist properties that enhance the release of norepinephrine, serotonin, and dopamine in mesocorticolimbic brain regions involved in reward, craving, and drug-seeking behavior (16, 27). This monoaminergic effect may mediate the observed reduction in methamphetamine craving.
A notable aspect of our study was the simultaneous reduction in both depression and craving in the intervention group. This finding is consistent with reports indicating that reductions in stimulant use are associated with improvements in depressive symptoms; for instance, a study by Voigt et al. demonstrated that decreased methamphetamine use correlated with reduced depressive symptoms (28). However, other studies have not found significant differences in depression between groups, highlighting the complexity of the relationship between substance use, depression, and pharmacotherapy.
Limitations of this study include the relatively small sample size and limited follow-up duration, which restrict generalizability. For example, the aforementioned systematic review emphasized the need for larger sample sizes and longer follow-up to confirm effects on depression (26). Additionally, recruitment from a limited and specific population may reduce the applicability of findings, although our sample consisted of general addiction treatment patients.
Clinically, these results suggest that mirtazapine could be a valuable option for MUD treatment, particularly in addiction programs with limited pharmacological alternatives. Nevertheless, safety monitoring, side effect management, and consideration of drug interactions are essential; while no serious adverse effects were observed in the present study, off-label use may carry risks such as overdose or suicidal behavior (29).
For future research, multicenter studies with larger sample sizes, extended follow-up periods, and combined pharmacotherapy and psychotherapy approaches are recommended. Further analyses using objective measures such as urine toxicology, quality of life assessments, and relapse rates are also necessary. Ongoing phase III trials, such as the Tina Trial, may provide more comprehensive data on the efficacy, safety, and administration of mirtazapine in clinical settings (30).
In conclusion, our findings support that mirtazapine, in addition to reducing methamphetamine craving, may also improve depressive symptoms in addiction treatment patients. This is particularly important given the limited pharmacological options currently available for this disorder. However, comprehensive evaluation, close safety monitoring, and further long-term studies are warranted.
5.1. Conclusions
The study findings indicated that mirtazapine significantly decreased craving, drug-seeking behavior, and depressive symptoms in the intervention group compared to controls. Side effects such as drowsiness, dry mouth, and appetite changes were not significantly different between groups, indicating good tolerability of the drug. These results highlight the potential of mirtazapine as an adjunctive treatment to reduce methamphetamine dependence symptoms and improve patients' psychological status.
5.2. Limitations
- The relatively small sample size and short follow-up period limit the generalizability of results.
- Baseline imbalance in depression scores between groups may confound analyses of specific drug effects.
- Recruitment from a limited population may reduce the applicability of findings to broader groups.