1. Background
Benign paroxysmal positional vertigo (BPPV) is the most common cause of periodic vertigo, usually beginning in the fifth and seventh decades of life (1), and accounts for about 30 percent of functional disorders of the peripheral vestibular system (2). In BPPV, the patient experiences short-term vertigo after head movements or rapid changes in head position (3). These symptoms worsen and occur rapidly, especially when rolling over in sleep or tilting the head back (4). After the symptoms resolve, the person can usually continue with their daily activities and there are no other neurological or auditory symptoms associated with this condition (5). The most common type of BPPV is primary or idiopathic BPPV, which has no known cause and accounts for 50 to 70 percent of cases (3, 6). It typically responds well to treatment. Secondary BPPV, caused by head trauma, accounts for 7 to 17 percent of cases (7). The cause of BPPV is the presence of basophilic deposits in the posterior semicircular canal (8). These deposits originate from the otolith organs (onicule and saccule) located in the vestibule of the ear (9). The calcium carbonate crystals present in these organs, which are essential for the proper function of non-accelerated linear movement receptors, make the canals sensitive to gravity and cause severe vertigo if they enter the semicircular canals (10). Recurrent BPPV is influenced by age, inner ear semicircular canals, and etiologic diseases (11). Also, Studies show the relationship between idiopathic BPPV and metabolic vitamin D disease (12) and calcium metabolic diseases (osteoporosis and osteopenia) (13). Patients with BPPV often have vitamin D deficiency (14). Given the high prevalence of BPPV and the problems it causes for the individual and his/her family, lack of proper treatment can lead to depression, job problems, lifestyle changes, and sleep disorders (15).
2. Objectives
Given the ambiguity in the exact pathophysiology of BPPV and the contradictory results regarding the effect of vitamin D, calcium, and phosphorus on this disease, this study aimed to investigate the relationship between the serum levels of these factors and BPPV.
3. Methods
3.1. Study Design
The current case-control study was conducted in ENT Clinic of Imam Khomeini Hospital (Kermanshah city) from 2019 to 2020.
3.2. Ethical Approval
The Kermanshah University of Medical Sciences Ethics Committee approved the study with the code IR.KUMS.REC.1399.1198.
3.3. Statistical Population and Participants
The study population consisted of people with BPPV with a definitive diagnosis in the case group and healthy people without a history of BPPV in the control group, matched in terms of age and gender. The samples were selected on a convenience basis. According to the study by Sen et al. (16), and considering a 76% prevalence of vitamin D deficiency in patients with BPPV and a 42% prevalence in the control group, the minimum sample size of 28 people in each group was calculated with a type I error probability (α) of 0.05. The inclusion criteria were patients with BPPV with complaints of severe positional vertigo, short attacks lasting 15 to 30 seconds, a history of previous vertigo, no vertigo without changing head position, a history of head trauma before the onset of vertigo, and vertigo that worsened at the beginning of the day and gradually relieved throughout the day. On the other hand, patients with vertigo with a history and recognizable maneuvers (even initial, recurrent, or with known causes such as head trauma), patients who were doubtful about the accuracy of their diagnosis or did not meet all the criteria for history and examination, cases suspected of other causes of vertigo (especially central nervous system involvement), and those taking anti-vertigo medications were excluded from the study.
3.4. Tools of Information Collection
After receiving written informed consent from the case and control groups, personal information was completed by the researcher (N. Farshchian). The data collection tool was a researcher-made checklist. The checklist included demographic information [age (year), gender (female/male), education (illiterate, primary or cycle, diploma, university), Body Mass Index (BMI, kg/cm2)]. The Dix-Hallpike diagnostic maneuver was used to diagnose vertigo in the present study. Also, to examine the serum levels of vitamin D, calcium and phosphorus, blood samples (5 cc) were taken from both groups. The vitamin D levels were measured using the high-performance liquid chromatography (HPLC), the calcium levels using the calorimetric method, and the phosphorus levels using the AAS method.
3.5. Data Analysis
Data analysis was performed after data collection and entry into SPSS software version 22. To describe quantitative data, central indicators [mean ± standard deviation (SD)] and to describe qualitative variables, frequency (percentage) were reported. After checking the normality of the data with the Kolmogorov-Smirnov test (assuming equal variances), the independent t-test was used to compare the quantitative data and the chi-square test was used to compare the qualitative data. If the data were not normal, the Mann-Whitney test was used. The significance level in all tests was considered to be 0.05.
4. Results
In this study, 56 people were examined. 28 of these people were in the case group and 28 were in the control group, whose average age was 44.1 ± 15.8 years and 44.2 ± 15.7 years, respectively. In both groups, there were 23 (82.1%) women and 5 (17.9%) men. The mean ± SD of BMI in the patient group were 31.26 ± 3.98, and in the control group were 26.13 ± 3.15, respectively. Also, the level of university education was 25% in the patients and 9.42% in the controls (Table 1).
| Variables | Case | Control |
|---|---|---|
| Age (y) | ||
| < 15 | 1 (3.6) | 0 (0.0) |
| 15 - 24 | 2 (7.1) | 3 (10.7) |
| 25 - 44 | 13 (46.4) | 12 (42.9) |
| 45 - 64 | 9 (32.1) | 10 (35.7) |
| > 65 | 3 (10.7) | 3 (10.7) |
| Education | ||
| Illiterate | 6 (21.4) | 3 (10.7) |
| Elementary | 4 (14.3) | 3 (10.7) |
| Middle | 4 (14.3) | 4 (14.3) |
| Diploma | 7 (25) | 6 (21.4) |
| University | 7 (25) | 12 (42.9) |
| BMI | ||
| < 18.5 | 0 (0.0) | 0 (0.0) |
| 18.5 - 24.5 | 9 (32.1) | 12 (42.9) |
| 24.5 - 29.9 | 14 (50.0) | 12 (42.9) |
| > 29.9 | 5 (17.9) | 4 (14.3) |
Abbreviation: BMI, Body Mass Index.
a Values are expressed as No. (%).
There was a significant difference between the mean serum vitamin D levels in the patient group (27.5 ± 15.3 pg/mL) and the control group (39.6 ± 15.8 pg/mL, P = 0.005). However, no significant difference was observed in serum calcium and phosphorus levels between the two groups (Table 2).
| Variables | Mean ± SD | Min - Max | P-Value |
|---|---|---|---|
| Vitamin D | 0.005 | ||
| Case | 27.5 ± 15.3 | 6.5 - 69 | |
| Control | 39.6 ± 15.8 | 16 - 81 | |
| Calcium | 0.366 | ||
| Case | 8.9 ± 0.3 | 8.2 - 9.6 | |
| Control | 8.8 ± 0.2 | 8.3 - 9.3 | |
| Phosphorus | 0.199 | ||
| Case | 3.1 ± 0.2 | 2.8 - 3.6 | |
| Control | 3.1 ± 0.3 | 2.4 - 3.6 |
Abbreviation: SD, standard deviation.
a The P-values < 0.05 was considered as significant with independent t-test and Mann-Whitney U test.
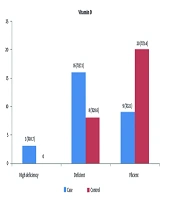
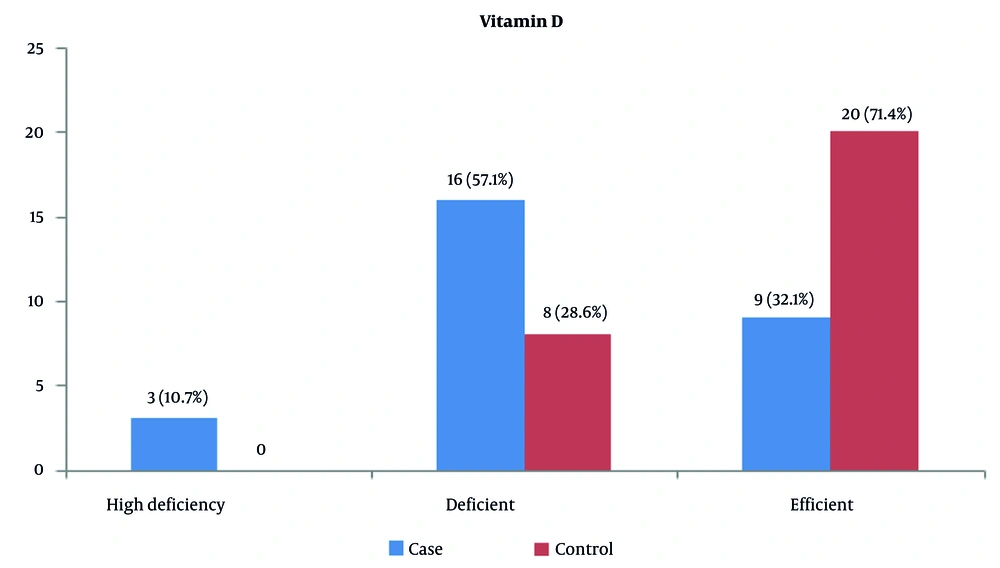
In the distribution of the severity of serum vitamin D levels, 20 (71.4%) of the control group and 9 (32.1%) of the patient group showed normal vitamin D levels. Severe vitamin D deficiency was observed in 3 (10.7%) of the patient group (Figure 1).
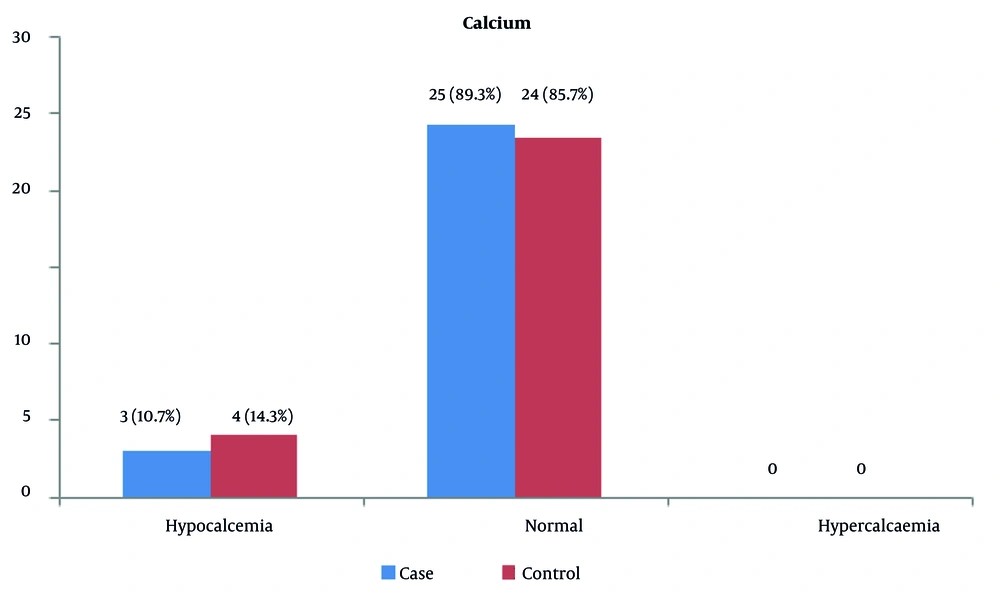
Calcium deficiency (hypocalcemia) was observed in 3 (7.10%) of the patients and 4 (3.14%) of the controls. Also, 25 (89.3%) of the patients and 24 (85.7%) of the controls had normal serum calcium levels (Figure 2).
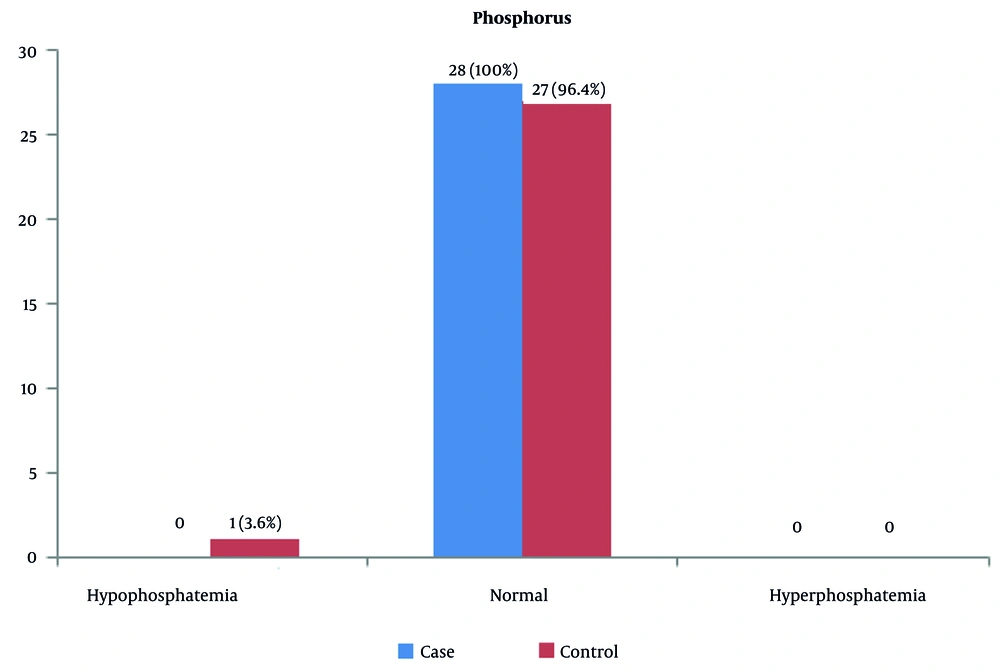
Serum phosphorus levels were normal in all patients and controls. Only one of the controls had phosphorus deficiency, and hyperphosphatemia was not observed in any of the people (Figure 3).
The results showed that in people over 65 years of age, the difference in mean serum vitamin D levels between the patient group (22.6 ± 4.4 pg/mL) and the control group (81.7 ± 36 pg/mL) was significant (P = 0.02, Table 3).
| Variables | Case | Control | P-Value |
|---|---|---|---|
| Vitamin D | |||
| < 15 | 25.0 ± 0.0 | 0.0 ± 0.0 | - |
| 15 - 24 | 33.0 ± 28.2 | 37.6 ± 6.4 | 0.999 |
| 25 - 44 | 28.3 ± 17.8 | 41.5 ± 20.6 | 0.099 |
| 45 - 64 | 27.1 ± 14.1 | 38.8 ± 14.3 | 0.092 |
| > 65 | 22.6 ± 4.4 | 36.7 ± 4.8 | 0.02 |
| Calcium | |||
| < 15 | 9.0 ± 0.0 | 0.0 ± 0.0 | - |
| 15 - 24 | 8.8 ± 0.2 | 9.1 ± 0.1 | 0.16 |
| 25 - 44 | 8.9 ± 0.3 | 8.8 ± 0.2 | 0.336 |
| 45 - 64 | 9.1 ± 0.2 | 8.8 ± 0.3 | 0.179 |
| > 65 | 8.6 ± 0.4 | 8.8 ± 0.3 | 0.539 |
| Phosphorus | |||
| < 15 | 3.2 ± 0.0 | 0.0 ± 0.0 | - |
| 15 - 24 | 3.1 ± 0.1 | 3.1 ± 0.3 | 0.898 |
| 25 - 44 | 3.1 ± 0.2 | 3.4 ± 0.2 | 0.28 |
| 45 - 64 | 3.1 ± 0.2 | 3.0 ± 0.2 | 0.143 |
| > 65 | 2.9 ± 0.0 | 3.1 ± 0.4 | 0.385 |
a The P-values < 0.05 was considered as significant with independent t-test and Mann-Whitney U test.
b Values are expressed as mean ± standard deviation (SD).
The mean serum vitamin D levels in the patient and control groups were 13.09 ± 24.56 pg/mL and 17.18 ± 39.49 pg/mL, respectively, which was statistically significant (P = 0.002). Also, the results also showed that the mean serum level of vitamin D among normal weight individuals in the control group was 42.08 ± 17.97 pg/mL and in the case group was 20.1 ± 11.19 pg/mL. The serum level of this vitamin in normal weight individuals was statistically significant (P = 0.005, Table 4).
| Variables | Case | Control | P-Value |
|---|---|---|---|
| Vitamin D | |||
| Sex | |||
| Female | 24.5 ± 5 | 39.4 ± 17.7 | 0.002 |
| Male | 41.3 ± 19.2 | 40.3 ± 8.2 | 0.841 |
| BMI | |||
| < 18.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | - |
| 18.5 - 24.5 | 20.1 ± 11.1 | 42.1 ± 17.9 | 0.005 |
| 24.5 - 29.9 | 32.7 ± 16.3 | 38.4 ± 16.4 | 0.391 |
| > 29.9 | 26.3 ± 15.8 | 35.9 ± 5.1 | 0.288 |
| Calcium | |||
| Sex | |||
| Female | 8.9 ± 0.3 | 8.8 ± 0.2 | 0.270 |
| Male | 8.9 ± 0.2 | 9.0 ± 0.2 | 0.727 |
| BMI | |||
| < 18.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | - |
| 18.5 - 24.5 | 8.9 ± 0.2 | 8.9 ± 0.3 | 0.896 |
| 24.5 - 29.9 | 9.3 ± 0.3 | 8.8 ± 0.1 | 0.072 |
| > 29.9 | 8.8 ± 0.2 | 8.9 ± 0.3 | 0.505 |
| Phosphorus | |||
| Sex | |||
| Female | 3.1 ± 0.2 | 3.3 ± 0.3 | 0.133 |
| Male | 3.4 ± 0.1 | 3.1 ± 0.2 | 0.723 |
| BMI | |||
| < 18.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | - |
| 18.5 - 24.5 | 3.1 ± 0.2 | 3.2 ± 0.3 | 0.191 |
| 24.5 - 29.9 | 3.1 ± 0.2 | 3.6 ± 0.2 | 0.239 |
| > 29.9 | 2.9 ± 0.1 | 3.5 ± 0.5 | - |
Abbreviation: BMI, Body Mass Index.
a The P-values < 0.05 was considered as significant with independent t-test and Mann-Whitney U test.
b Values are expressed as mean ± standard deviation (SD).
5. Discussion
The present study was conducted to investigate the relationship between serum levels of vitamin D, calcium, and phosphorus with BPPV. The results of the study showed that the mean serum vitamin D level in the patient group was significantly lower than that in the control group (P = 0.005), while no significant difference was observed in mean calcium and phosphorus between the two groups. In line with the results of a recent study, the study by AlGarni et al. showed that the vitamin D level in the patient groups was significantly reduced (14). The study by Jeong et al. showed that serum vitamin D levels were low in patients with BPPV, regardless of age, gender, BMI, and decreased bone density (17). The studies by Cikrikci Isik et al. (18) and Buki et al. (19) did not show a significant difference in vitamin D levels between patients and controls. Also, Parham et al. (20) did not find a significant relationship between BPPV, vitamin D, and calcium. Karatas et al. (21) also reported that osteoporosis and vitamin D deficiency are not risk factors for BPPV. The association of vitamin D with BPPV is not equally reproducible across populations and studies and may be influenced by demographic, geographic, nutritional, measurement methods, and control for confounding factors.
In this study, serum vitamin D levels in women in the patient group were lower than in men, and a statistically significant relationship was observed between serum vitamin D levels and gender (female). Comparing serum vitamin D, calcium, and phosphorus levels in different age groups showed that in the patient group, the mean serum vitamin D level in the age group over 65 years was significantly lower than in the control group (P = 0.02). The study by Rhim (12) also showed that most people with BPPV are women and their average age is 50.3 years. Atli et al. (22) stated that vitamin D deficiency is more common in older women and that the level of this vitamin decreases with age. The study by Sen et al. (16) also showed a high prevalence of osteoporosis and vitamin D deficiency after menopause in people with BPPV. Unlike previous studies, the study by Cikrikci Isik et al. (18) did not show a significant difference between age, vitamin D and calcium levels in the two groups studied. As a result, women are more likely to suffer from vitamin D deficiency than men, and the prevalence of this deficiency in Kermanshah province is high, approximately 40 to 60 percent. Considering that most of the patient participants in this study were women with an average age close to menopause, lower serum vitamin D levels in women compared to men is an expected result. Since our study population consists of hospital patients, and women usually visit the hospital more often than men to follow up on their physical condition, and on the other hand, the use of sunscreens and special clothing by Iranian women prevents them from receiving enough vitamin D through sunlight, it is not possible to reach a definitive conclusion solely by relying on these studies.
The results of the study showed that the mean BMI in both groups was almost similar and most people were overweight. The findings of Karatas et al. (21) also showed that the mean BMI of BPPV patients was in the overweight range. Overweight and obesity, regardless of comorbidities, increase the prevalence of vitamin D deficiency, and this deficiency can affect weight gain (23, 24). The present study did not examine the effect of seasonal changes on vitamin D, but its time period was appropriate for this purpose. It is suggested that future studies with a longer time period compare vitamin D levels in different seasons. Also, to cover individual influential variables, it is necessary to use a larger sample size. Considering the selection of patients and the diagnostic method, it is assumed that age, gender, and other confounding variables are equally distributed and their influence on BPPV is negligible. However, selection bias in case-control studies cannot be ignored. To gain more in-depth information, identify confounding factors, and investigate the effect of comorbidities such as hypertension and diabetes, prospective randomized studies, especially at the laboratory and molecular levels, are necessary to obtain physiopathological information of the disease.
5.1. Conclusions
In summary, the study of serum calcium, phosphorus, and vitamin D levels in patients with BPPV showed that vitamin D is significantly associated with this disease. Vitamin D deficiency is more common in women, especially in the age group near menopause, and can aggravate the disease BPPV.
5.2. Limitations
The main limitation of this study is its small sample size, which cannot be representative of the situation of patients with BPPV in the community. In addition, the lack of information on the number of BPPV recurrences, duration of the disease, type of BPPV, and duration of vertigo recovery after the Hallpike maneuver are other weaknesses of this study that should be considered in future studies. Also, information on common regional diseases (such as metabolic or vascular diseases) that can cause symptoms similar to vertigo was not collected, and only patients' statements about medical history were used. Although diseases associated with vertigo (especially diseases of the central nervous system) were excluded from the study based on exclusion criteria, the possible association of vertigo with comorbidities cannot be ignored.