1. Background
Nitisinone, also known as 2-[2-nitro-4-(trifluoromethyl)benzoyl]-1,3-cyclohexanedione (NTBC), is a 4-hydroxyphenylpyruvate dioxygenase inhibitor that plays a crucial role in the tyrosine catabolic pathway. It is primarily used to treat hereditary tyrosinemia type 1 (HT1, Figure 1) (1, 2). Genetic mutations in fumarylacetoacetate hydrolase, the final enzyme involved in tyrosine catabolism, lead to HT1 (3, 4). The deficiency of this enzyme results in excessive urinary excretion of homogentisic acid (HGA) and the buildup of toxic metabolites like maleylacetoacetate and fumarylacetoacetate in the tissues of affected individuals. These harmful metabolites can cause damage to the liver and kidneys (5). Infants with HT1 commonly experience acute liver failure, cirrhosis, neurological crises characterized by pain and paralysis, and renal tubular dysfunction leading to hypophosphatemic rickets (6). Nitisinone binds effectively to 4-hydroxyphenylpyruvate dioxygenase, thereby reducing the flow of metabolites through the tyrosine catabolic pathway and lowering the production of toxic substances (7). Treatment with nitisinone has been shown to effectively prevent acute hepatic and neurological crises in compliant patients with HT1. It has also been demonstrated to be safe, effective, and well-tolerated for both short- and long-term use in these patients.
Various analytical methods have been employed to determine this drug, including high-performance liquid chromatography (HPLC) for the analysis of nitisinone (8, 9), reverse-phase high-performance liquid chromatography (RP-HPLC) for the determination of nitisinone in dosage forms (10, 11), and mass spectrometry (12, 13). Most of the time, HPLC and LC/MS methods are more expensive and complex than spectrophotometric and spectrofluorimetric methods. Spectrophotometry is regarded as the most convenient analytical technique due to its simplicity, affordability, and widespread availability in many quality control laboratories (14, 15). Spectrofluorimetry is also a sensitive and simple technique with good analytical selectivity, which in most cases can improve the limit of detection (LOD) (16).
2. Objectives
As far as we are aware, there have been no previous reports on the spectrophotometric and spectrofluorimetric analysis of nitisinone in dosage forms. This study presents the development of both spectrofluorimetric and spectrophotometric techniques for determining nitisinone in capsule dosage forms. In continuation of some other works in our laboratory, fluorescein was used to form a charge transfer complex between nitisinone and fluorescein at a pH of 6 for the spectrofluorimetric method.
3. Methods
3.1. Apparatus
An RF-5000 spectrofluorometer (Shimadzu, Japan) was used for the measurement of fluorescence spectra. The slit widths for both the excitation and emission monochromators were set to 5 nm. For all spectrophotometric analyses, a double-beam UV-vis spectrophotometer (160A, Shimadzu, Japan) was employed. The pH levels of various solutions were determined using a Metrohm pH meter from Switzerland.
3.2. Chemicals and Reagents
Nitisinone was generously supplied by Osvah Pharmaceutical Company, Tehran, Iran (Batch No.: 9009011). The nitisinone capsules (Batch No.: 9009011), each containing 2 mg of nitisinone, were also produced by Osvah Pharmaceutical Company. Fluorescein was purchased from Merck. Additionally, all the chemicals and solvents utilized were of analytical quality.
3.3. Standard Solutions
A stock standard solution was prepared by dissolving 2 mg of nitisinone in 100 mL of methanol in a volumetric flask. Dilution was done with the same solvent, and finally, a series of standard solutions (0.1 to 5 μg/mL) was prepared. These solutions remained stable for at least one week when stored in the refrigerator. The Britton-Robinson buffer consisted of 0.04 M of boric acid, phosphoric acid, and acetic acid, which was adjusted to a pH range of 1 - 12. A 0.006 M fluorescein solution was prepared in methanol.
3.4. General Procedure
3.4.1. Method I
Aliquots of nitisinone solution (0.1, 0.2, 0.5, 1, 1.5, 2, 2.5, and 3 μg/mL) were transferred into 10 mL volumetric flasks. Next, 1 mL of 0.006 M fluorescein and 2 mL of buffer (pH = 6) were added to each. The solutions were then brought to volume with methanol. The fluorescence intensity was measured at 517 nm using excitation wavelengths of 265 and 326 nm, with a blank solution (containing all substances except nitisinone) as the reference. The difference in the fluorescence intensity of the blank and sample solutions (ΔF = F° - F, where F° is the fluorescence of the blank and F is the fluorescence of the nitisinone-fluorescein complex) was plotted versus the final concentration of the drug (μg/mL).
3.4.2. Method II
A volume of 1 mL of nitisinone solution (0.1, 0.2, 0.5, 1, 2, 3, 4, and 5 μg/mL) was placed into 10 mL volumetric flasks, and the flasks were filled to the mark with methanol. The absorbance of these solutions was recorded at 256 nm, using the solvent as a blank reference.
3.4.3. Method III
After following the procedure mentioned in method II, the derivative spectra of the standard solution were recorded for the first to fourth derivative orders at different Δλ values. The suitable wavelengths in the first to fourth order derivative spectra, which showed better intensity, were selected for the analysis.
3.5. Accuracy and Precision
The precision and accuracy of the proposed methods for both within-day and between-day measurements were determined by examining three sets of nitisinone standard solutions over one day and three consecutive days.
3.6. Analysis of Capsules
The contents of ten nitisinone capsules were carefully ground into a fine powder. A precisely measured amount of this powder, corresponding to 2 mg of nitisinone, was dissolved in 50 mL of methanol. The mixture was sonicated for 20 minutes, filtered, and then brought to volume with methanol (100 mL). Aliquots of the diluted solution and a standard nitisinone solution of equivalent concentration were analyzed for nitisinone content using the proposed spectrophotometric and spectrofluorimetric methods.
4. Results and Discussion
Nitisinone is not soluble in water and is very poorly soluble in ethanol, but it is completely soluble in methanol and acetonitrile. Since acetonitrile is not regarded as an environmentally friendly solvent, methanol was chosen for further experiments due to its stability and classification as a greener alternative (17).
4.1. Method I
4.1.1. Fluorescence Spectrum
Nitisinone does not exhibit native fluorescence. However, when it interacts with fluorescein, a charge transfer complex is formed, which causes the fluorescence of fluorescein to diminish. Figure 2 illustrates the fluorescence spectra for both fluorescein and the nitisinone-fluorescein complex. Fluorescein exhibits its highest intensity (λem) at 517 nm when excited at 265 nm and 326 nm. Upon charge transfer complex formation of nitisinone with fluorescein, the fluorescence was quenched. The value of the fluorescence quenching (ΔF = F° - F) was plotted versus the concentration of nitisinone.
4.1.2. Optimization of Reaction Variables
4.1.2.1. Effect of pH
Fluorescein is classified as a weak acid with a pKa of 6.4, and its ionization equilibrium results in pH-dependent absorption and emission within the pH range of 5 to 9. The effect of pH on the ΔF of the fluorescein-nitisinone complex was studied using a Britton-Robinson buffer solution that covered a wide pH range (1 - 12), keeping all other variables constant. The drug precipitated when the pH was raised above 6. In a pH = 6 weak acidic medium, the carboxyl group at the 2’ position dissociates, and the univalent anion is associated with the NO2 group of nitisinone with the positive charge to form an ion pair complex. As shown in Figure 3, when the pH is adjusted to 6, the maximum fluorescence quenching of the labeled analyte is achieved at 517 nm (λem). Hence, a buffer solution with a pH value of 6 was used in this study.
4.1.2.2. The Fluorescein/Nitisinone Ratio
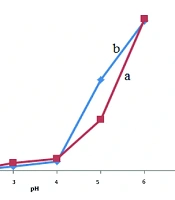
The effect of fluorescein concentration on the fluorescence intensity of the fluorescein-nitisinone complex was investigated using the limiting logarithmic method with λex = 265 nm and λex = 326 nm and λem = 517 nm (Figure 4). The results reveal that the optimum fluorescein/nitisinone ratio was 1:1.
Based on the findings regarding the effect of pH and the ratio of the fluorescein-nitisinone complex, the proposed structure of the ion-pair complex is shown in Figure 5. The complex formation results in the fluorescence quenching of fluorescein without changing the excitation and emission wavelengths (Figure 5).
4.1.2.3. Stability of the Complex
The fluorescence intensity of the fluorescein-nitisinone complex was monitored over a 3-hour period, with measurements taken every 30 minutes, and again after 24 and 48 hours. The findings indicated that there was no change in fluorescence intensity during the initial 3 hours, and after 24 hours, the variations in fluorescence were no greater than 5% (under daylight and room temperature conditions).
4.1.3. Validation of the Spectrofluorimetric Method
The proposed spectrofluorimetric method was tested for various validation criteria such as linearity, precision, accuracy, LOD, limit of quantification (LOQ), etc. Under the optimized experimental conditions, the relationship between fluorescence intensity and nitisinone concentration was assessed and found to be linear within the range of 0.1 - 3 μg/mL. The linear regression equation was Y = 109.04X + 11.38 and Y = 117.21X + 13.15 for excitation λ of 265 nm and 326 nm and emission λ of 517 nm, respectively, where Y is ΔF = F° - F and X is the concentration of the drug in μg/mL. The limits of detection and quantification were found to be 0.03 and 0.09 μg/mL for excitation λ of 265 nm and 0.01 and 0.04 μg/mL for excitation λ of 326 nm, respectively (Table 1).
| Parameters | Ex = 265; Em = 517 | Ex = 326; Em = 517 |
|---|---|---|
| Linearity | 0.10 - 3.00 µg/mL | 0.10 - 3.0 µg/mL |
| LOD | 0.03 µg/mL | 0.01 µg/mL |
| LOQ | 0.09 µg/mL | 0.04 µg/mL |
| Regression equation | Y = 109.04; X + 11.38 | Y = 117.21; X + 13.15 |
| SD of slope | 1.01 | 0.49 |
| RSD of slope | 0.93 | 0.42 |
| SD of intercept | 1.31 | 1.28 |
| Correlation coefficient | 0.9971 | 0.9970 |
Abbreviations: LOD, limit of detection; LOQ, limit of quantification.
The accuracy and precision of the method for both within-day and between-day assessments were tested by measuring various concentrations (0.2, 1.5, and 3 μg/mL) on four separate occasions within a single day and over three consecutive days. The findings are presented in Table 2.
| Added (µg/mL) | Within-Day (n = 4) | Between-Day (n = 12) | ||||
|---|---|---|---|---|---|---|
| Found ± SD (µg/mL) | CV (%) | Error (%) | Found ± SD (µg/mL) | CV (%) | Error (%) | |
| λex = 265 nm; λem = 517 nm | ||||||
| 0.20 | 0.202 ± 0.005 | 2.475 | 1.000 | 0.203 ± 0.006 | 2.956 | 1.500 |
| 1.50 | 1.508 ± 0.009 | 0.596 | 0.553 | 1.526 ± 0.026 | 1.704 | 1.733 |
| 3.00 | 2.970 ± 0.008 | 0.269 | -1.000 | 2.950 ± 0.019 | 0.644 | -1.667 |
| λex = 326 nm; λem = 517 nm | ||||||
| 0.2 | 0.202 ± 0.002 | 0.990 | 1.000 | 0.206 ± 0.007 | 3.398 | 3.000 |
| 1.50 | 1.532 ± 0.009 | 0.587 | 2.133 | 1.534 ± 0.011 | 0.717 | 2.267 |
| 3.00 | 2.951 ± 0.020 | 0.678 | -1.633 | 2.949 ± 0.016 | 0.543 | -1.700 |
4.2. Methods II and III
4.2.1. Spectrophotometric Method
The absorbance spectrum of nitisinone was recorded in the range of 200 - 400 nm using methanol as the solvent. Nitisinone shows a maximum wavelength at 256 nm (Figure 6). Additionally, derivative spectra of nitisinone in different orders (first to fourth) were recorded to find the best intensity for nitisinone with minimum noise. Comparing different order derivative spectra of nitisinone, it was observed that the maximum intensity at 323 nm in the second-order spectrum and at 305 nm and 359 nm in the third-order spectrum are suitable for the determination of nitisinone.
4.3. Validation of the Spectrophotometric Method
The suggested method demonstrated linearity within the concentration ranges of 0.1 - 5 μg/mL for zero-order spectrophotometry and 0.5 - 5 μg/mL for derivative spectrophotometry of nitisinone (Table 3). The detection and quantification limits for nitisinone were calculated using the formulas LOD = 3SD/b and LOQ = 10SD/b, where SD refers to the residual standard deviation obtained from the linear regression and b signifies the slope of the regression line (18).
| Parameters | (λ = 256 nm) | 2D (Δλ = 24.5) (λ = 323 nm) | 3D (Δλ = 28) (λ = 305 nm) | 3D (Δλ = 31.5) (λ = 359 nm) |
|---|---|---|---|---|
| Linearity range (µg/mL) | 0.10 - 5.00 | 0.50 - 5.00 | 0.50 - 5.00 | 0.50 - 5.00 |
| LOD (µg/mL) | 0.03 | 0.02 | 0.01 | 0.02 |
| LOQ (µg/mL) | 0.10 | 0.08 | 0.03 | 0.05 |
| Regression equation | Y = 0.079; X - 0.004 | Y = 0.047; X - 0.009 | Y= -0.0107; X + 0.029 | Y = 0.086; X - 0.026 |
| SD of slope | 8.100 × 10-4 | 3.920 × 10-4 | 2.810 × 10-5 | 4.590 × 10-4 |
| Slope RSD (%) | 1.03 | 0. 83 | 0.26 | 0.53 |
| SD of intercept | 9.83 × 10-4 | 3.530 × 10-3 | 7.12 × 10-3 | 7.370 × 10-3 |
| Correlation coefficient | 0.9997 | 0.9976 | 0.9991 | 0.9969 |
Abbreviations: LOD, limit of detection; LOQ, limit of quantification.
The accuracy and precision of these methods were assessed for both within-day and between-day variations at three distinct concentrations, with testing conducted on the same day (n = 3) and across three separate days (n = 9). The results are summarized in Table 4. The relative standard deviation (< 2.4) and the relative error (< 2.5) of the proposed methods were found to be suitable for the routine analysis of nitisinone.
| Added (µg/mL) | Within-Day (n = 3) | Between-Day (n = 9) | ||||
|---|---|---|---|---|---|---|
| Found ± SD (µg/mL) | CV (%) | Error (%) | Found ± SD (µg/mL) | CV (%) | Error (%) | |
| Zero-order; λ = 256 nm | ||||||
| 0.20 | 0.205 ± 0.002 | 0.976 | 2.500 | 0.205 ± 0.005 | 2.440 | 2.500 |
| 2.00 | 1.963 ± 0.004 | 0.204 | -1.850 | 1.985 ± 0.019 | 0.957 | -0.750 |
| 5.00 | 5.060 ± 0.014 | 0.277 | 1.200 | 5.040 ± 0.022 | 0.437 | 0.080 |
| 2D (Δλ = 24.5); λ = 332 nm | ||||||
| 1.00 | 1.019 ± 0.015 | 1.470 | 1.900 | 1.010 ± 0.016 | 1.584 | 1.000 |
| 3.00 | 2.950 ± 0.063 | 2.143 | -1.644 | 2.982 ± 0.053 | 1.777 | -0.600 |
| 5.00 | 5.089 ± 0.0161 | 0.314 | 1.780 | 5.088 ± 0.042 | 0.825 | 1.800 |
| 3D (Δλ = 28); λ = 305 nm | ||||||
| 1.00 | 0.991 ± 0.007 | 0.706 | -0.900 | 0.994 ± 0.006 | 0.604 | -0.600 |
| 3.00 | 2.997 ± 0.031 | 1.034 | -0.100 | 2.989 ± 0.040 | 1.338 | -0.366 |
| 5.00 | 4.977 ± 0.024 | 0.482 | -0.460 | 4.991 ± 0.031 | 0.621 | -0.180 |
| 3D (Δλ = 31.5); λ = 359 nm | ||||||
| 1.00 | 0.990 ± 0.003 | 0.303 | -1.000 | 0.997 ± 0.012 | 1.203 | -0.300 |
| 3.00 | 2.996 ± 0.007 | 0.234 | -0.133 | 3.044 ± 0.074 | 2.431 | 1.460 |
| 5.00 | 5.015 ± 0.033 | 0.658 | 0.300 | 5.011 ± 0.029 | 0.579 | 0.220 |
4.4. Application of the Proposed Methods
These methods were successfully applied to analyze nitisinone in capsules. The concentration of the product determined by the proposed methods was in good agreement with the labeled claim. The outcomes of the suggested methods were statistically analyzed in comparison to the reference HPLC method (10) (Table 5), and there was no significant difference between them (P < 0.05).
| Methods | Nitisinoe (mg) | Recovery (%) |
|---|---|---|
| Spectrofluorimetry; λex = 265; λem = 517 nm | 2.056 ± 0.055 | 102.80 |
| Spectrofluorimetry; λex = 326; λem = 517 nm | 2.057 ± 0.022 | 102.85 |
| Spectrophotometry; zero-order; λ = 256 nm | 1.976 ± 0.008 | 98.80 |
| Spectrophotometry; 2D (Δλ = 24.5); λ = 332 nm | 1.968 ± 0.014 | 98.40 |
| Spectrophotometry; 2D (Δλ = 28); λ = 305 nm | 1.958 ± 0.024 | 97.90 |
| Spectrophotometry; 3D (Δλ = 31.5); λ = 359 nm | 1.982 ± 0.016 | 99.10 |
| HPLC | 1.999 ± 0.024 | 99.95 |
Abbreviation: HPLC, high-performance liquid chromatography.
4.5. Relative Recovery
The percentage of recovery, assessed by applying the standard addition technique, was in the range of 96.5 - 100.7 for all the different proposed methods (Table 6).
| Methods | Relative Recovery (%) |
|---|---|
| Spectrofluorimetry; λex = 265; λem = 517 nm | 98.11 ± 0.75 |
| Spectrofluorimetry; λex = 326; λex = 517 nm | 96.50 ± 0.95 |
| Spectrophotometry; zero-order; λ = 256 | 99.05 ± 0.82 |
| Spectrophotometry; 2D (Δλ = 24.5); λ = 332 nm | 98.82 ± 2.08 |
| Spectrophotometry; 2D (Δλ = 28); λ = 305 nm | 97.89 ± 1.69 |
| Spectrophotometry, 3D (Δλ = 31.5); λ = 359 nm | 100.66 ± 2.10 |
4.6. Comparison with Other Reported Methods
The amount of nitisinone in dosage forms has been investigated in recent years using other methods, such as HPLC and reverse-phase HPLC. Table 7 pre3sents the results of these studies makes the suitability of the method discussed for the present study more evident.
Abbreviations: LOD, limit of detection; LOQ, limit of quantification; HPLC, high-performance liquid chromatography; RP-HPLC, reverse-phase high-performance liquid chromatography.
4.7. Conclusions
A spectrofluorimetric method based on the formation of an ion-pair complex of nitisinone with fluorescein at pH 6 was developed to determine nitisinone in capsules. A simple and precise zero, second, and third-order derivative spectrophotometric measurement was also presented. The derivative spectrophotometric method used in this study can serve as an alternative in cases of existing interferences. The results demonstrated that there was no significant difference between the proposed and standard HPLC methods. Nevertheless, the standard HPLC method remains of great importance in complex matrices due to its specificity. Ultimately, it seems that both spectrophotometric and spectrofluorimetric methods could be used for the determination of nitisinone in pharmaceutical dosage forms.



![Limiting logarithmic plot for the ratio of nitisinone and fluorescein; a, log (ΔF) versus log [nitisinone]; b, log (ΔF) versus log [fluorescein]; A, λex = 265 nm, λem = 517 nm; and B, λex = 326 nm, λem = 517 nm Limiting logarithmic plot for the ratio of nitisinone and fluorescein; a, log (ΔF) versus log [nitisinone]; b, log (ΔF) versus log [fluorescein]; A, λex = 265 nm, λem = 517 nm; and B, λex = 326 nm, λem = 517 nm](https://services.brieflands.com/cdn/serve/3170d/16b41fedbac03c8156f48b4da7ce1d11b6bf1579/jrps-13-1-160785-i004-preview.webp)

