1. Introduction
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a rare yet severe and potentially life-threatening hypersensitivity reaction to certain medications, including anticonvulsants, sulfonamides, and allopurinol (1, 2). Initially recognized in the mid-20th century following the introduction of hydantoin-based anticonvulsants, DRESS syndrome has since been documented as a significant challenge due to its unpredictable nature and multisystem involvement (3, 4). Its clinical presentation commonly includes fever, widespread skin rash, lymphadenopathy, hematologic abnormalities, and dysfunction of internal organs such as the liver, kidneys, and lungs. However, the heterogeneity in symptom severity and systemic involvement complicates its diagnosis and management (1). Recent studies also suggest that the reactivation of viral infections, such as human herpesvirus-6 (HHV-6), may play a crucial role in the pathogenesis of DRESS syndrome, further adding to the complexity of its clinical course (5).
Among anticonvulsant medications, aromatic antiepileptic drugs are a known trigger for DRESS syndrome. Lamotrigine, an aromatic antiepileptic widely prescribed for focal and tonic-clonic seizures, Lennox-Gastaut syndrome, and bipolar disorder, was previously considered to carry a lower risk of inducing DRESS compared to other drugs in its class (6, 7); however, recent pharmacovigilance data suggest a significantly elevated reporting risk (8). Recent case reports highlight the variability of lamotrigine-induced DRESS presentations, including atypical manifestations such as severe renal involvement, delayed eosinophilia, and multi-organ failure, emphasizing the need for clinician awareness and early detection (9, 10). Despite its rarity, the syndrome can have fatal outcomes, especially in cases where diagnosis is delayed or management is suboptimal (11).
This report describes a unique case of lamotrigine-induced DRESS syndrome in a patient with intellectual disability, presenting with atypical multi-organ dysfunction. Through a detailed examination of this case and a review of the existing literature, this study aims to shed light on the diverse clinical manifestations, diagnostic challenges, and therapeutic approaches for lamotrigine-induced DRESS syndrome. By highlighting these aspects, the report underscores the importance of vigilance and timely intervention to improve patient outcomes, especially in vulnerable populations.
2. Case Presentation
We present the case of a 40-year-old man with a known history of intellectual disability and a six-year history of polypharmacy for the management of behavioral disorders. His prescribed chronic medications included sodium valproate 500 mg twice daily, perphenazine 4 mg daily, thioridazine 50 mg daily, quetiapine 100 mg daily, chlordiazepoxide 10 mg daily, propranolol 20 mg twice daily, and trazodone 50 mg at bedtime. Notably, three weeks prior to the onset of symptoms, lamotrigine was introduced as an adjunctive therapy, starting at 25 mg/day and titrated to 75 mg/day (25 mg three times daily) until his admission to our facility. No other new medications were initiated during this period.
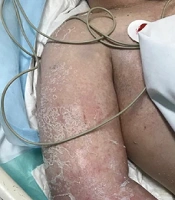
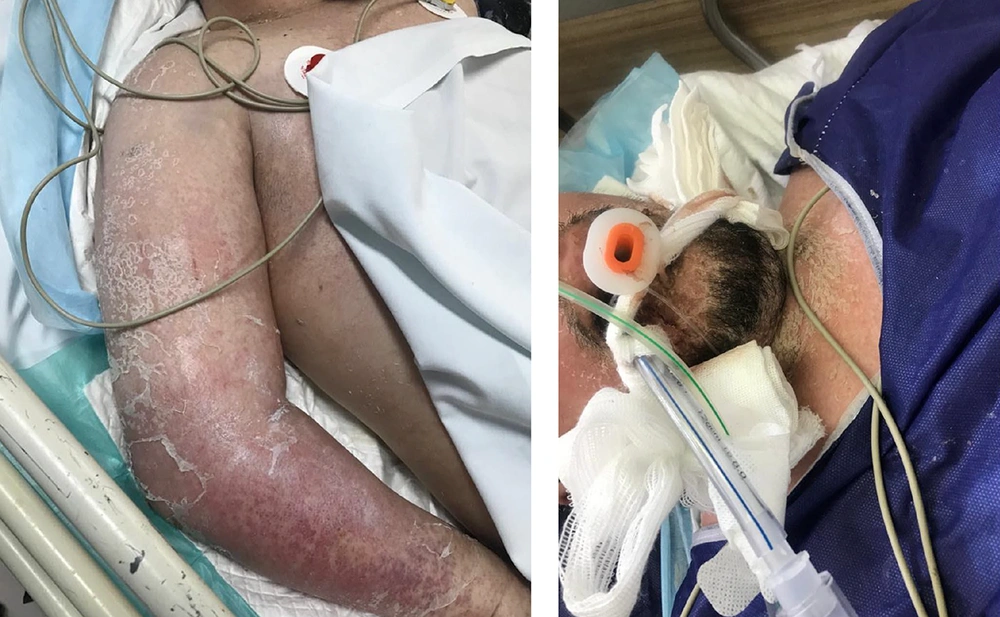
Upon presentation, the patient reported fever and a widespread skin rash. Physical examination revealed an extensive diffuse maculopapular rash with scaling, prominently affecting the face, neck, and extremities (Figure 1). Additional findings included facial edema and the presence of blisters, particularly on the palms, though no mucosal involvement was observed. Vital signs indicated an oral temperature of 39.2°C, tachycardia with a heart rate of 110 beats per minute, a respiratory rate of 14 breaths per minute, hypotension with a blood pressure of 80/55 mmHg, and an oxygen saturation level of 88% on room air. Due to a significant decline in consciousness, the patient required urgent intubation and mechanical ventilation. Cardiac evaluation was performed to assess potential myocarditis, including electrocardiogram (ECG) and measurement of cardiac enzymes (troponin I and CK-MB). Both ECG and cardiac biomarkers were within normal limits, indicating no evidence of cardiac involvement.
Initial laboratory investigations demonstrated marked elevations in liver enzymes, with aspartate aminotransferase (AST) at 1272 U/L, alanine aminotransferase (ALT) at 557 U/L, alkaline phosphatase (ALP) at 80 U/L, and total bilirubin at 2.2 mg/dL. Additional findings included hyperkalemia (serum potassium 5.9 mEq/L), hyperphosphatemia (serum phosphorus 8.7 mg/dL), hypoalbuminemia (serum albumin 2.9 g/dL), hyperuricemia (serum uric acid 8.9 mg/dL), and an elevated blood urea level of 243 mg/dL. Renal impairment was evident with a serum creatinine level of 4.1 mg/dL. Strikingly, there were substantial elevations in lactate dehydrogenase (LDH) at 9851 U/L, creatine phosphokinase (CPK) at 19,500 U/L, and C-reactive protein (CRP) at 91 U/L. Despite these abnormalities, the white blood cell count remained within normal limits (9000/mm3), with no evidence of eosinophilia (1.6%). However, atypical lymphocytes were present (13.1%).
Imaging studies revealed pleural effusion on chest CT, while abdominal ultrasound showed no abnormalities in the liver, spleen, or kidneys. Extensive microbiological testing, including blood, urine, and cerebrospinal fluid cultures, yielded negative results. Serological testing for hepatitis B, hepatitis C, and human immunodeficiency virus (HIV) also returned negative. Based on the clinical presentation and laboratory findings, the patient was diagnosed with DRESS syndrome, confirmed by a RegiSCAR score of 6, meeting the criteria for a definitive case (Table 1). This diagnosis underscores the critical importance of early recognition and intervention in preventing further systemic complications.
| Variables | Score |
|---|---|
| Fever ≥ 38.5°C | 0 |
| Enlarged lymph nodes | Unknown (0) |
| Eosinophilia | 0 |
| Atypical lymphocytes | 1 |
| Skin involvement (> 50% body surface area) | 1 |
| Skin rash suggesting DRESS | 1 |
| Biopsy suggesting DRESS | 0 |
| Organ involvement (≥ 2 organs) | 2 |
| Resolution ≥ 15 d | 0 |
| Evaluation of other potential causes | None positive and ≥ 3 negative = 1 |
| ANA | |
| Blood culture | |
| Serology for HAV/HBV/HCV/ Chlamydia/Mycoplasma pneumonia | |
| Other serology/PCR | |
| Total score | 6 |
Abbreviation: DRESS, drug reaction with eosinophilia and systemic symptoms.
a Final score < 2: No case; final score 2 - 3: Possible case; final score 4 - 5: Probable case; final score > 5: Definite case.
b Definitive DRESS diagnosis is defined as a RegiSCAR score ≥ 6. The patient’s score was 6, confirming a definitive case.
2.1. Treatment
Upon confirming the diagnosis of DRESS syndrome, immediate discontinuation of lamotrigine was undertaken as the first and most critical step. Topical medications were initiated to manage the skin manifestations, and a pulse therapy of methylprednisolone at a dosage of 500 mg daily was administered intravenously for three consecutive days. Following the pulse therapy, the patient was transitioned to oral prednisolone at a dosage of 50 mg per day. Given the severity of renal impairment, intermittent dialysis sessions were conducted every other day to manage the patient’s metabolic derangements and prevent further complications.
2.2. Outcome and Follow-up
Initially, the patient showed promising signs of clinical improvement. Three days after the initiation of methylprednisolone therapy, extubation was successfully performed as his respiratory function stabilized. Liver enzyme levels, which were significantly elevated on admission, normalized during this period. Marked reductions were also observed in LDH and CPK levels, while serum creatinine demonstrated a gradual but consistent declining trend, indicating partial recovery of renal function. Despite these improvements, the patient’s neurological status remained unchanged, with no significant improvement in consciousness.
Tragically, on the fifth day following pulse therapy, the patient experienced an abrupt decline. A sudden onset of pancytopenia was observed, with laboratory findings showing a hemoglobin level of 7.1 g/dL, a white blood cell count of 3000/mm3, and a platelet count of 50,000/mm3. Simultaneously, the patient developed respiratory distress, necessitating re-intubation and mechanical ventilation. The CRP levels escalated dramatically to 213 U/L, raising suspicion of sepsis as a potential secondary complication. In response, broad-spectrum antibiotics were initiated, and steroid therapy was discontinued to minimize immunosuppression. Despite these aggressive interventions, the patient’s condition continued to deteriorate, and he succumbed to his illness.
This case underscores the complexity and unpredictable nature of DRESS syndrome, particularly when associated with severe multi-organ involvement. It highlights the critical importance of early recognition and treatment, as well as the challenges in managing complications such as pancytopenia and sepsis that can arise even after initial improvement.
3. Discussion
The DRESS is a rare but severe adverse drug reaction with a reported mortality rate ranging between 10% and 20% (4, 12). Lamotrigine is among the most frequently implicated agents (13), and its association with multisystem involvement underscores the necessity of prompt recognition and intervention. A recent large-scale pharmacovigilance study using the U.S. FDA Adverse Event Reporting System (FAERS) highlighted that lamotrigine carries a statistically significant reporting risk for DRESS syndrome, with a reporting odds ratio (ROR) of 10.29 (95% CI: 9.66 - 10.96) (8).
Although the precise pathophysiology of DRESS remains unclear, proposed mechanisms include aberrant T-cell activation, impaired drug detoxification pathways, and genetic predisposition (4). The current case adds to the growing literature on lamotrigine-induced DRESS syndrome by highlighting an unusually severe presentation involving multi-organ failure, including hepatic and renal dysfunction, respiratory failure, and hematologic collapse. A distinctive feature in our case was the absence of eosinophilia — traditionally regarded as a hallmark of DRESS syndrome. However, although eosinophilia is a key element in the syndrome’s name, it is not universally present. Studies have shown that up to one-third of DRESS patients may exhibit normal eosinophil counts, while lymphocyte abnormalities such as atypical lymphocytosis are often more prominent in these cases (14-16). In our review of 34 cases of lamotrigine-induced DRESS (Table 2), eosinophilia was reported in approximately 82% of patients, whereas 18% lacked this feature, reinforcing the notion that its absence does not preclude the diagnosis. These findings underscore the importance of comprehensive clinical assessment and the application of standardized diagnostic tools such as the RegiSCAR scoring system. The terminology itself can be misleading; since eosinophilia is not an indispensable feature, some authors have proposed the alternative term drug-induced hypersensitivity syndrome (DIHS) to more accurately reflect the condition’s pathophysiological basis without implying the mandatory presence of eosinophilia (17).
| First Authors; y | Gender/Age (y) | Latency (d) | Systemic Involvement | Eosinophilia | EBV, CMV and HHV Reactivation | Concomitant Use of Valproate | Dose | Outcome |
|---|---|---|---|---|---|---|---|---|
| Demas and Shaikh (18); 2024 | Female/18 | ~21 (3 wk) | Skin, liver (hepatitis), and lymphadenopathy | Mild (0.6 - 0.57 ×103/µL) | EBV: Not reported; CMV: Not reported; HHV-6: Not reported | No | 100 mg daily | Full recovery following 16-day course of oral corticosteroids |
| Larocca Gonzalez et al. (10); 2024 | Female/47 | ~21 (after dose increase) | Skin, liver (AST/ALT ↑, cholestasis), and facial edema | Yes (9%) | EBV: Negative; CMV: Negative; HHV-6: Not reported | Not reported | 25 → 75 mg/day | Complete resolution after oral corticosteroids |
| Bruning et al. (9); 2024 | Female/17 | 21 | Acute kidney injury | Yes | EBV: Negative; CMV: Negative; HHV-6/8: Negative | No | 25 mg/day | Partial recovery |
| Jaquez-Quintana et al. (19); 2024 | Male/72 | Not specified (recent switch from valproate) | Skin, liver (cholestasis), lymphadenopathy, GI tract (eosinophilic colitis), and CMV reactivation | Yes | EBV: Not reported; CMV: Not reported; HHV-6: Not reported | Yes (recently switched from valproate) | Not specified | Full recovery in 5 days without steroids or antivirals |
| Al-Hasan et al. (20); 2024 | Female/29 | ~21 | Skin, lymphadenopathy, liver, gallbladder (acalculous cholecystitis), and EBV reactivation | Yes (12%) | EBV: Positive; CMV: Not reported; HHV-6: Not reported | No | 25 mg BID → 50 mg BID | Full recovery after lamotrigine discontinuation and oral steroids |
| Duan et al. (21); 2023 | Female/18 | 12 | Skin (rash), lymphadenopathy | Yes (10.0%) | EBV: Not reported; CMV: Not reported; HHV-6: Not reported | Yes | 25 mg BID | Recovery within 1 month after IV methylprednisolone treatment |
| Doan et al. (22); 2023 | Female/39 | ~5 (after dose increase) | Skin, liver, heart (myocarditis), and thyroid | Yes (1.8 K/µL, 16%) | EBV: Negative; CMV: Negative; HHV-6: Not reported | No | Increased from 75 to 100 mg | Death (cardiac arrest due to eosinophilic myocarditis) |
| Kiblawi et al. (23); 2022 | Male/43 | 14 | Skin, liver, platelets, and EBV reactivation | Not reported | EBV: Positive; CMV: Negative; HHV-6: Not reported | Yes | Not specified | Full recovery in ~12 days |
| Vrhovski et al. (24); 2022 | Female/52 | 2 | Skin, liver (transaminitis), and fever | Absent | EBV: Not reported; CMV: Not reported; HHV-6: Not reported | Not reported | Not specified (first-time administration) | Recovery with corticosteroids |
| Abdelnabi et al. (25); 2022 | Male/28 | 21 | Kidney, skin, and liver | Yes (18%) | EBV: Positive; CMV: Positive (past infection); HHV-6: Not reported | No | 100 mg/day | Recovered with corticosteroids |
| Clark and St Clair (26); 2022 | Female/46 | 24 | Skin, fever, and lymphadenopathy | Yes | EBV: Not reported; CMV: Not reported; HHV-6: Not reported | Not reported | 100 mg BID | Partial recovery, intermittent symptoms, and skin discomfort |
| Kounatidis et al. (27); 2022 | Female/26 | ~30 | Skin, lymphadenopathy, spleen, and pancytopenia | Yes (530 cells/mm3) | EBV: Negative; CMV: Negative; HHV-6/8: Negative | No | Not specified | Full remission in 10 days with corticosteroids |
| Shah et al. (28); 2022 | Male/52 | 21 | Skin, liver, pulmonary, and kidneys | Yes (20%) | EBV: Negative; CMV: Negative; HHV-6: Not reported | No | 100 mg/day | Recovered with corticosteroids |
| Packard et al. (29); 2022 | Male/45 | 14 | Skin, liver, and kidney | Yes | Full-text unavailable | No | 200 mg/day | Recovered after stopping both drugs (lamotrigine and lacosamide) |
| Engin et al. (30); 2021 | Male/13 | 14 | Liver (AST/ALT ↑), hematologic (PLT↓), and skin (rash) | Yes (7%) – borderline | EBV: Not reported; CMV: Not reported; HHV-6: Not reported | Yes | Not specified (Lamotrigine added to valproate) | Full recovery with corticosteroids |
| Lin et al. (31); 2021 | Female/7 | 14 | Liver and lungs | Yes (10%) | EBV: Negative; CMV: Not reported; HHV-6: Not reported | Yes | Initial: 50 mg/day | Cured (steroids+immunosuppressants) |
| Bozca et al. (32); 2020 | Female/31 | 21 | Skin (purpuric lesions), oral mucosa, and thyroid function abnormality | No | EBV: Not reported; CMV: Not reported; HHV-6: Not reported | Yes | 25 mg/day | Recovered with corticosteroids |
| Afonso et al. (33); 2020 | Male/38 | 28 | Liver and skin | Yes (15%) | EBV: Not reported; CMV: Not reported; HHV-6: Not reported | Yes | 200 mg/day | Recovered with corticosteroids |
| Parsi and Daniel (34); 2020 | Female/32 | 14 | GI tract (eosinophilic colitis), skin, and fever | Yes (43%) | EBV: Not reported; CMV: Not reported; HHV-6: Not reported | Not reported | Not reported | Full recovery after systemic corticosteroids |
| Lameiras et al. (1); 2019 | Male/50 | 28 | Skin, liver (↑AST/ALT), lymphadenopathy, and fever | Yes (30%) | EBV: Negative; CMV: Negative; HHV-6/7: Negative | Yes | 25 → 50 mg/day | Full recovery after 10-week corticosteroid therapy |
| Jeremic and Ostojic (35); 2018 | Female/55 | 56 | Skin, fever, and hematologic (lympho-, thrombocytopenia) | Absent | EBV: Not reported; CMV: Not reported; HHV-6: Not reported | No | Not reported | Resolution after lamotrigine withdrawal and corticosteroids |
| Agarwal et al. (36); 2017 | Female/22 | 21 | Skin (rash), liver (↑ALT/AST), and fever | Yes (13%) | EBV: Not reported; CMV: Not reported; HHV-6: Not reported | No | Not reported | Recovery after drug withdrawal and antihistamines |
| Stephan et al. (37); 2016 | Female/30 | 40 | Skin, lymph nodes, liver, spleen, and hematologic (CD30+infiltrate mimicking lymphoma) | Not reported | EBV: Not reported; CMV: Not reported; HHV-6: Not reported | Not reported | 50 mg daily | Full recovery after oral corticosteroids |
| Oriolo et al. (38); 2016 | Male/25 | 35 | Skin (erythroderma), lymph nodes, liver (↑ALT), kidney (↑creatinine), hematologic, and fever | Yes (3.8 ×109/L) | EBV: Negative; CMV: Not reported; HHV-6: Negative | No | 25 → 50 → 200 mg/day | Full recovery after corticosteroids and drug withdrawal |
| Balaban and Ninkovic-Baros (39); 2015 | Female/44 | 30 | Skin, liver (ALT ↑), pancreas (↑ urine amylase), and hematologic | Yes (13.8%, up to 15%) | EBV: Negative; CMV: Negative; HHV-6: Not reported | No | Not reported | Complete resolution after corticosteroid therapy |
| Kocaoglu et al. (40); 2013 | Male/6 | 10 | Skin, lymph nodes, and fever (no internal organ involvement) | Yes (26%) | EBV: Negative; CMV: Negative; HHV-6: Negative | Yes | Not reported | Complete recovery with antihistamines only |
| Ginory et al. (16); 2013 | Female/17 | 21 | Skin, liver (↑AST/ALT/bilirubin), lymph nodes, fever, facial edema, mucosa, and splenomegaly | Yes (0.85×109/L) | EBV: Not reported; CMV: Not reported; HHV-6: Not reported | No | 25 mg twice daily | Recovery with IV methylprednisolone, taper of prednisone |
| Singer et al. (41); 2013 | Female/21 | 14 | Skin, lymph nodes, liver, kidney, fever, hematologic; post-DRESS: Hypothyroidism, and type 1 diabetes | Yes (10%) | EBV: Not reported; CMV: Not reported; HHV-6: Not reported | No | Not reported | Refractory DRESS, improved with IVIG; later developed autoimmune sequelae |
| Bicknell et al. (42); 2012 | Female/50 | 30 | Skin (85% BSA), oral mucosa, lymph nodes, fever, and hematologic changes | Yes (5.3%) | EBV: Not reported; CMV: Not reported; HHV-6: Not reported | No | Not reported | Recovery after steroids (topical+oral) |
| Naveen et al. (43); 2012 | Male/12 | 20 | Skin, liver (↑AST/ALT), lymph nodes, fever, and facial edema | Yes (19%) | EBV: Not reported; CMV: Not reported; HHV-6: Not reported | Yes | 25 mg → 50 mg/day | Complete recovery with corticosteroids |
| Pereira De Silva et al. (11); 2011 | Female/32 | 45 | Skin, liver (↑ALT), fever, and lymph nodes | Yes (1606 cells/µL) | EBV: Not reported; CMV: Not reported; HHV-6: Not reported | Yes | 200 mg twice daily | Complete remission with corticosteroids |
| Female/21 | 18 | Skin, fever, lymphadenopathy, and eosinophilia | Yes (1419 cells/µL) | Yes | 100 mg four times daily | |||
| Female/15 | 30 | Skin, liver (↑ALT), fever, and lymphadenopathy | Yes (3800 cells/µL) | Yes | 75 mg twice daily | |||
| Roquin et al. (44); 2010 | Male/75 | 40 | Skin, liver (↑AST/ALT), kidney (renal failure), pancreas (acute pancreatitis), lymph nodes, and fever | Yes | EBV: Not reported; CMV: Not reported; HHV-6: Positive | No | Up to 100 mg at day 30 | Initial improvement with corticosteroids; later death due to nosocomial pneumonia |
| Amante et al. (45); 2009 | Female/21 | 18 to 38 | Skin (rash), liver (fulminant failure), lymph nodes, fever | Yes | EBV: Negative; CMV: Negative; HHV-6: Not reported | No | Not reported | Liver transplant due to fulminant hepatic failure |
| Eshki et al. (46); 2009 | Female/25 | 25 | Pneumonitis, infection, shock | Not reported | EBV: Not reported; CMV: Not reported; HHV-6: Not reported | Not reported | Not reported | ICU admission, survived after corticosteroid therapy |
Abbreviations: EBV, Epstein-Barr virus; CMV, cytomegalovirus; HHV-6, human herpesvirus-6; AST, aspartate aminotransferase; ALT, alanine aminotransferase; DRESS, drug reaction with eosinophilia and systemic symptoms; IVIG, intravenous immunoglobulin.
Several studies have demonstrated that reactivation of certain viruses — particularly members of the Herpesviridae family — plays a significant role in the pathogenesis of DRESS syndrome (5, 23). Among these, HHV-6 is the most commonly implicated, followed by HHV-7, cytomegalovirus (CMV), and Epstein-Barr virus (EBV) (16). Reactivation of these viruses has not only been linked to the onset and progression of DRESS but is also associated with more severe clinical outcomes. Consequently, routine screening for viral reactivation is recommended as part of the diagnostic evaluation and prognostic assessment in patients with suspected DRESS (5).
While we were unable to evaluate HHV-6 reactivation in our patient due to resource limitations, our review of lamotrigine-induced DRESS cases revealed that viral reactivation was inconsistently assessed and poorly reported. Among the 34 cases analyzed, EBV reactivation was documented in only 3 cases, CMV in 2, and HHV-6 in 3, whereas the majority of reports did not evaluate or mention viral reactivation at all. Specifically, testing for EBV and HHV-6 was absent in approximately 60% of the cases. These findings highlight a significant gap in diagnostic workup and reflect the underutilization of viral screening in clinical settings, despite the recognized role of viral reactivation in the pathogenesis and prognosis of DRESS syndrome.
Interestingly, in our review, HHV-6 reactivation was reported in only three cases, and one of them was associated with a fatal outcome. While definitive conclusions cannot be drawn due to limited reporting, this observation may suggest a lower frequency of HHV-6 reactivation in lamotrigine-induced DRESS compared to other drug triggers. Further studies are needed to clarify this potential association.
In comparison with previously reported cases of lamotrigine-induced DRESS, our patient presented with a particularly severe phenotype, characterized by early respiratory failure, multiorgan dysfunction, and ultimately a fatal outcome. While the average latency period in the reviewed cases ranged from 14 to 30 days, our patient developed symptoms approximately 21 days after lamotrigine initiation, which is consistent with the typical onset window for DRESS (13). Notably, valproate was co-administered, and lamotrigine was titrated to 75 mg/day within a relatively short time frame — an approach that may have increased the risk of hypersensitivity (43).
Of the 34 published cases, the male-to-female ratio was approximately 1:2, consistent with prior observations suggesting a higher incidence among females. While the majority of patients experienced favorable outcomes following timely corticosteroid therapy, fatal outcomes were reported in at least three cases, primarily those with extensive multiorgan involvement, paralleling the course observed in our patient. Hepatic involvement was the most frequently reported systemic manifestation, occurring in over 85% of cases, followed by renal, pulmonary, gastrointestinal, and hematologic involvement. Our patient demonstrated markedly elevated liver enzymes (ALT 557 U/L), consistent with prior reports of severe hepatic injury.
A review of the reported cases of lamotrigine-induced DRESS (Table 2) further supports these observations. The majority occurred in young to middle-aged females, with a latency of 2 - 4 weeks after lamotrigine initiation. Skin and liver were the most frequently affected organs, followed by hematologic, renal, and pulmonary manifestations. Concomitant valproate therapy was reported in approximately one-third of cases and may increase susceptibility. Most patients recovered after lamotrigine withdrawal and corticosteroid therapy; however, a few fatal cases have been documented, primarily due to fulminant hepatic failure or myocarditis.
In comparison, our patient — a 40-year-old man with intellectual disability and polypharmacy — developed unusually severe multi-organ involvement including hepatic injury, renal failure requiring dialysis, respiratory compromise, and later pancytopenia. Despite timely initiation of high-dose corticosteroids and supportive measures, the course was fatal. This contrast underscores the unpredictable nature of DRESS and emphasizes the importance of careful monitoring in patients with multiple comorbidities or concurrent psychotropic therapies.
Although renal involvement in DRESS is less common than hepatic injury and typically mild (47), both our case and that of Bruning et al. demonstrate that it can be severe and life-threatening. Our patient developed acute anuric renal failure requiring dialysis, while Bruning et al. reported biopsy-proven acute interstitial nephritis and tubular necrosis in a teenager with DRESS (9). These cases highlight the need for early recognition and close renal monitoring in patients with suspected DRESS.
Nevertheless, despite early initiation of high-dose pulse corticosteroids, our patient’s condition deteriorated, illustrating the limitations of immunosuppressive therapy in cases with rapidly progressive multiorgan failure. The patient's history of intellectual disability and long-standing polypharmacy may have contributed to the atypical and severe clinical course observed. These factors can complicate the recognition of early symptoms and potentially increase susceptibility to hypersensitivity reactions. While direct evidence linking intellectual disability to drug-induced immunologic reactions is limited, this association warrants further investigation. In such vulnerable populations, clinicians may consider initiating treatment with lower doses and ensuring close clinical monitoring to minimize adverse outcomes.
Timely identification and immediate withdrawal of the offending drug remain the cornerstone of DRESS management (2). Our treatment approach included high-dose intravenous methylprednisolone followed by oral prednisolone. Although this regimen led to initial improvements in hepatic and respiratory function, the patient experienced an abrupt deterioration marked by pancytopenia, respiratory failure, and presumed sepsis. Unlike the gradual improvement commonly reported following corticosteroid tapering, this sudden decline underscores the unpredictable nature of DRESS and the challenges of managing multisystem involvement.
According to recent treatment guidelines, systemic corticosteroids are considered the first-line therapy in DRESS, particularly in patients with severe organ involvement, including elevated liver enzymes, renal dysfunction, or pulmonary compromise. Prednisolone at a dose of 1 mg/kg/day with gradual tapering over 4 - 6 weeks is typically recommended (17). In severe or refractory cases, adjunctive therapies such as intravenous immunoglobulin (IVIG), cyclosporine, or mycophenolate mofetil may be considered, although their efficacy remains under investigation (2, 12). Additionally, in patients with confirmed viral reactivation, antivirals such as ganciclovir may be beneficial (17).
A limitation of our report is the absence of immunohistochemistry (IHC) or other pathological studies for the skin lesions, which could have provided additional insights into the immunopathogenesis of lamotrigine-induced DRESS syndrome. Future case reports and studies incorporating IHC or other histopathological analyses may help improve understanding of the cellular mechanisms underlying severe drug hypersensitivity reactions.
3.1. Conclusions
This case highlights the diverse clinical spectrum and potential severity of lamotrigine-induced DRESS syndrome. The absence of eosinophilia, severe renal impairment, and poor steroid responsiveness underscore the diagnostic and therapeutic challenges of the condition. Special consideration should be given to vulnerable populations such as those with intellectual disability. Early detection, prompt drug withdrawal, and individualized treatment remain essential to improving patient outcomes.