1. Background
Prangos and Cachrys are two genera belonging to the deep-rooted Apiaceae family and are distributed worldwide, particularly in the Irano-Turanian phytogeographic area (1, 2). Prangos platychlaena Boiss. and Cachrys scabra (Fenzl) Meikle are perennial herbaceous taxa that are endemic to the Iraqi Kurdistan region (3, 4) and are locally known as “Chewre” and “Xwerik”, respectively, in Kurdish. Different parts of both plants have been used in folk medicine across numerous communities for treating a variety of illnesses, including antispasmodic, antihemorrhoidal, rheumatic, carminative, diuretic, gout, and wound-healing conditions (1, 2, 5).
The widespread use of Prangos and Cachrys in folk medicine, together with the recognition of their terpene-rich essential oils (EOs) by the pharmaceutical industry (3, 6), underscores the need for a deeper understanding of their various properties, including phytochemical, toxicological, and pharmacological characteristics (1, 2, 7).
2. Objectives
This study aimed to investigate the antioxidant and antimicrobial potency of EOs obtained from P. platychlaena and C. scabra, belonging to the Apiaceae family, which are indigenous to Iraqi Kurdistan.
3. Methods
3.1. Plant Material
Leaves and flowers of P. platychlaena and Cachrys scabra were collected from the Halgurd Mountain in the Kurdistan Region of Iraq during June - July 2023 (altitude: 2268 m; Figure 1). The plant material was identified by Assistant Professor Abdullah Shukur Sardar, a botanist from the Department of Biology, Salahaddin University-Erbil. The collected plant parts were sorted, dried, and ground into a fine powder. The powdered samples were stored in air-tight containers at room temperature (25.00 ± 2.00°C), protected from light until further use.
3.2. Isolation of the Essential Oils
The EOs were extracted using the hydro-distillation method with a Clevenger apparatus for three hours. The obtained EOs were dried over anhydrous sodium sulfate and stored in dark vials at 4°C until analysis. The EO of P. platychlaena was pale yellowish in color, while that of C. scabra appeared clear.
3.3. DPPH Radical Scavenging Activity
The antioxidant activity was measured using the DPPH free radical scavenging assay, following the procedure described by Dahal et al. (8), with slight modifications. First, 100 µL of DPPH (90 µM) solution prepared in methanol (95% v/v) was added to each well. Then, 100 µL of serial dilutions of the EOs (0.08 - 10 mg/mL), as well as the positive control (vitamin C), were added to 100 µL of DPPH in methanol. The reaction mixture was shaken vigorously and incubated for 30 minutes. The absorbance of the solutions was then measured at 516 nm, and the radical scavenging activity (%) was calculated using the following equation: Radical scavenging activity% = (Abs blank - Abs sample)/(Abs blank) × 100.
The IC50 value was determined as the concentration of the sample required to inhibit 50% of the initial DPPH radical.
3.4. ABTS+ Radical Scavenging Assay
The ABTS+ assay was performed according to the method described by Zengin et al. (9). The ABTS+ cationic radical was generated by mixing 100 µL of 2.45 mM potassium persulfate with 10 mL of 7 mM ABTS solution. The resulting mixture was incubated at room temperature in the dark for 16 hours. The ABTS+ solution was then diluted with methanol to obtain an absorbance of 0.70 ± 0.002 at 734 nm.
Next, 100 µL of the ABTS+ cationic radical solution was mixed with 100 µL of serial concentrations of EOs (0.08 - 10 mg/mL) or vitamin C (positive control). After a 30-minute incubation in the dark, the absorbance of the mixture was measured at 734 nm. The antiradical activity of the samples was expressed as the percentage inhibition of the ABTS+ radical, calculated using the same equation described previously for the DPPH assay.
3.5. Antimicrobial Activity
Nine bacterial strains were tested for antimicrobial activity, including six gram-positive species — Staphylococcus aureus (ATCC 25923), S. epidermidis (ATCC 12228), Enterococcus faecalis (ATCC 15753), Bacillus pumilus (PTCC 1274), B. cereus (PTCC 1015), and B. subtilis (ATCC 9372) — and three gram-negative species — Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27852), and Klebsiella pneumoniae (ATCC 3583). Tetracycline (30 µg), gentamicin (10 µg), and ampicillin (10 µg) were used as positive controls.
3.6. Disc Diffusion Assay
The antimicrobial activity of the EOs was tested against pathogenic strains using the agar disc diffusion method described by Dahal et al. (8). Sterile Whatman filter paper discs (6 mm in diameter) were placed in the center of Petri dishes and impregnated with 20 µL of the EOs at a concentration of 1 mg/mL. The inoculated Petri dishes were incubated at 37°C for 24 - 48 hours. Following incubation, the diameters of the growth inhibition zones (GIZ) were measured in millimeters (mm). The inhibition zones (IZ) were categorized as very strong (> 20 mm), strong (10 - 20 mm), moderate (5 - 10 mm), or weak (< 5 mm) (10).
3.7. Microdilution Assay
The EOs that exhibited notable IZ against indicator strains in the agar disc diffusion assay were further analyzed quantitatively to determine their minimum inhibitory concentration (MIC) values. The broth microdilution method was employed to determine MIC, following the procedure described by Rahman et al. (11). Various concentrations of EOs (120 - 3.5 mg/mL) were added to 5 mL of appropriate broth tubes containing 107 CFU/mL of live bacterial cells. To ensure uniform dispersion of the oil throughout the broth, the tubes were incubated on a shaker incubator and then examined for visible microbial growth. The MIC was defined as the lowest concentration of EOs showing no visible bacterial growth after incubation.
3.8. Statistical Analysis
All antioxidant and antibacterial assays were performed in triplicate. The results are presented as mean values ± standard deviation (SD). The IC50 values for antioxidant tests were calculated from linear regression equations using Microsoft Excel 2021.
4. Results and Discussion
4.1. DPPH Radical-Scavenging Assay of Essential Oils
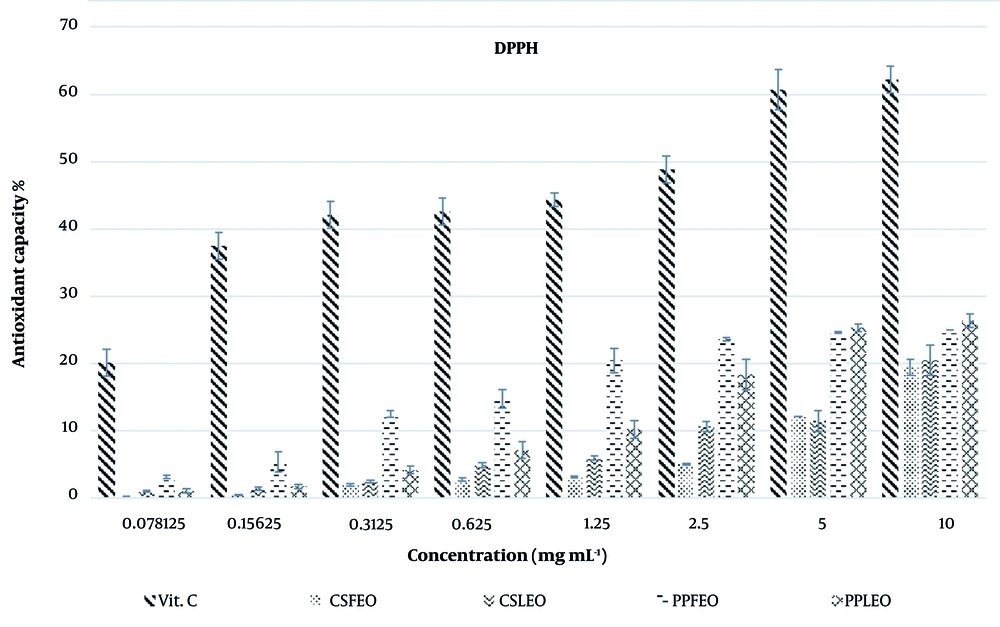
The DPPH radical scavenging activity of the EOs from P. platychlaena and C. scabra is presented in Figure 2 and Table 1. Both the EOs and the positive control exhibited dose-dependent inhibition of the DPPH radical. All tested samples demonstrated significant antioxidant potential, with IC50 values of 16.55 ± 0.27 mg/mL and 25.34 ± 0.73 mg/mL, which were comparable to that of the positive control (vitamin C, IC50 = 4.76 ± 0.10 mg/mL). PPLEO and PPFEO exhibited markedly higher antioxidant activity than Cachrys scabra leaf essential oil (CSLEO) and Cachrys scabra flower essential oil (CSFEO).
| Sample No. | Sample Code | IC50 ± SD [mg mL-1] | |
|---|---|---|---|
| DPPH | ABTS | ||
| 1 | CSFEO | 25.34 ± 0.73 | 9.95 ± 0.16 |
| 2 | CSLEO | 25.09 ± 1.31 | 10.80 ± 0.41 |
| 3 | PPFEO | 19.39 ± 0.09 | 6.63 ± 0.25 |
| 4 | PPLEO | 16.55 ± 0.27 | 10.90 ± 0.34 |
| 5 | Vitamin C | 4.76 ± 0.10 | 1.81 ± 0.07 |
Abbreviations: SD, standard deviation; CSFEO, Cachrys scabra flower essential oil; CSLEO, Cachrys scabra leaf essential oil.
Within the concentration range tested (0.0785 - 10 mg/mL), the antioxidant potential of the EOs followed the order: PPLEO > PPFEO > CSLEO > CSFEO.
4.2. ABTS Radical Scavenging Activity of Essential Oils
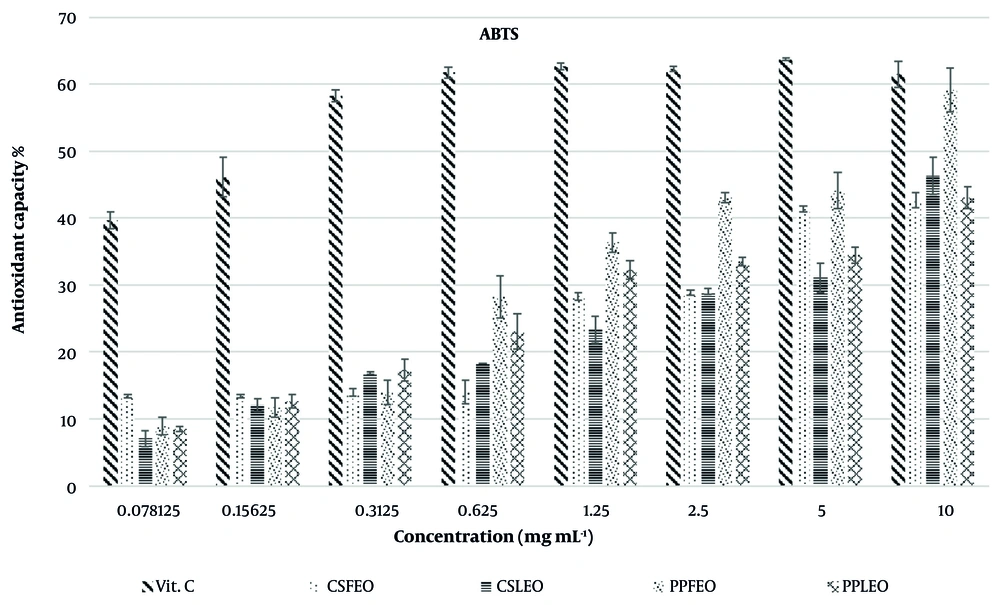
As shown in Figure 3, the ABTS radical scavenging activity of the EOs and vitamin C was concentration-dependent, similar to the DPPH assay. At the highest concentration (10 mg/mL) of all EOs, the antioxidant capacity ranged between 41.43 ± 1.60% and 59.79 ± 3.30%, while that of vitamin C (positive control) was 63.75 ± 1.98% (Table 1). Table 1 also presents the concentrations of the EOs required to inhibit 50% of the ABTS radicals, expressed as IC50 values.
Among the tested oils, PPLEO exhibited the lowest anti-ABTS radical activity with an IC50 value of 10.90 ± 0.34 mg/mL, while PPFEO demonstrated the highest anti-radical potency with an IC50 value of 6.63 ± 0.25 mg/mL. Considering the tested concentration range (0.0785 - 10 mg/mL), the antioxidant potential of the EOs followed the order: PPFEO > CSFEO > CSLEO > PPLEO.
Our findings are consistent with previous studies investigating the inhibitory effects of EOs from Prangos and Cachrys genera against DPPH and ABTS radical scavenging (5, 12). In a study conducted in Iraq, Rahman and Safar (3) reported that the EOs of C. scabra exhibited higher DPPH free radical scavenging activity compared to other extracts, with IC50 values ranging from 6 to 8.6 mg/mL. Similarly, Matejic et al. (13) from Serbia demonstrated significant free radical scavenging properties in an aqueous extract of C. cristata fruits, with an IC50 value of 1.784 mg/mL, followed by methanolic extracts of fruits and aerial parts with IC50 values of 3.347 and 4.058 mg/mL, respectively.
Rahman et al. (12) from Iraq found that the petroleum ether extract of the flower of P. platychlaena exhibited higher DPPH antioxidant scavenging activity (IC50 = 0.19 ± 0.01 mg/mL) than the root, leaves, and stem. Furthermore, Baghiani et al. (5) from Algeria reported that the crude extract of C. libanotis roots displayed strong DPPH scavenging activity, with an IC50 of 0.41 ± 0.01 mg/mL, followed by the ethyl acetate extract (IC50 = 0.57 ± 0.01 mg/mL). In addition, P. uechtritzii EO from Turkey, studied by Zengin et al. (9), demonstrated higher activity for DPPH free radical scavenging (IC50 = 1.74 ± 0.10 mg TE/g) among three Prangos species, while P. heyniae EO exhibited the most significant ABTS scavenging activity (IC50 = 92.99 ± 1.29 mg TE/g).
4.3. Antibacterial Activity of Essential Oils
The antimicrobial activity of the EOs from P. platychlaena and C. scabra against nine bacterial strains is summarized in Table 2. All tested bacterial strains were sensitive to both P. platychlaena and C. scabra EOs, with GIZ diameters ranging from 10 ± 0.5 to 20 ± 1.8 mm. However, P. aeruginosa (ATCC 27852) showed complete resistance, displaying no inhibition by any of the oils.
| Samples | Bacillus pumilus (PTCC 1274) | B. subtilis (ATCC 9372) | Staphylococcus aureus (ATCC 25923) | B. cereus (PTCC 1015) | Enterococcus faecalis (ATCC 15753) | S. epidermidis (ATCC 12228) | Klebsiella pneumonia (ATCC 3583) | Escherichia coli (ATCC 25922) | Pseudomonas aeruginosa (ATCC 27852) |
|---|---|---|---|---|---|---|---|---|---|
| PPFEO | |||||||||
| IZ ± SD (mm) | 11 ± 1.32 b | 12 ± 0.5 | 18 ± 0.5 | 12 ± 0.5 | 11 ± 1.8 | 18 ± 10 | 10 ± 0.5 | 17 ± 0.5 | - |
| MIC (mg mL-1) | > 15 c | 15 | 7.5 | 15 | 15 | 7.5 | 15 | 7.5 | - |
| PPLEO | |||||||||
| IZ ± SD (mm) | 12 ± 0.5 | 12 ± 1 | 18 ± 1.5 | 11 ± 1.32 | 11 ± 1.8 | 17 ± 0.87 | 12 ± 1.73 | 14 ± 1.73 | - |
| MIC (mg mL-1) | 15 | 15 | 7.5 | 15 | > 15 | 7.5 | 15 | 15 | - |
| CSLEO | |||||||||
| IZ ± SD (mm) | 18 ± 0.87 | 10 ± 1.32 | 20 ± 1.8 | 11 ± 0.5 | 14 ± 0.5 | 10 ± 0.87 | 20 ± 1.73 | 14 ± 0.5 | - |
| MIC (mg mL-1) | 7.5 | 15 | 7.5 | 15 | 15 | 15 | 7.5 | 15 | - |
| CSFEO | |||||||||
| IZ ± SD (mm) | 17 ± 1 | 12 ± 0.87 | 14 ± 1 | 12 ± 0.87 | 11 ± 0.87 | 14 ± 2 | 18 ± 1.8 | 14 ± 0.87 | - |
| MIC (mg mL-1) | 7.5 | 15 | 15 | 15 | > 15 | 15 | 7.5 | 15 | - |
| Tetracycline d | |||||||||
| IZ ± SD (mm) | - | 14 ± 12.12 | 20 ± 1.73 | Nt | Nt | 34 ± 1.73 | Nt | 0 | Nt |
| MIC (mg mL-1) | - | 3.2 | 3.2 | - | - | 1.6 | - | Nt | - |
| Gentamicin e | |||||||||
| IZ ± SD (mm) | - | - | - | Nt | Nt | 0 | Nt | 23 ± 1.8 | Nt |
| MIC (mg mL-1) | - | Nt | Nt | - | - | Nt | - | 3.2 | - |
| Ampicillin e | |||||||||
| IZ ± SD (mm) | 15 ± 0.5 | 14 ± 0.87 | 13 ± 0.87 | Nt | Nt | 19 ± 0.87 | Nt | 12 ± 0.87 | Nt |
| MIC (mg mL-1) | 15 | 15 | 15 | - | - | 15 | - | 15 | - |
Abbreviations: IZ, inhibition zones; SD, standard deviation; MIC, minimum inhibitory concentration; CSLEO, Cachrys scabra leaf essential oil; CSFEO, Cachrys scabra flower essential oil; Nt, not tested.
a Values are expressed as mean ± SD.
b Zone of inhibition (in mm) includes diameter of the disc (6 mm) with concentration 1 mg/disc.
c MIC values as mg mL-1, (-): Inactive, (7 - 13): Moderately active, (> 14): Highly active.
d Tested at 30 μg/disc.
e Tested at 10 μg/disc.
The flower and leaf EOs of P. platychlaena exhibited notable activity against three pathogenic strains — S. aureus, S. epidermidis, and E. coli — with IZ diameters ranging from 14 ± 1.73 to 18 ± 1.0 mm. For C. scabra, both CSLEO and CSFEO displayed moderate to strong inhibitory effects against all tested strains (except P. aeruginosa), with IZ values between 10 ± 0.87 and 20 ± 1.8 mm.
The MIC results indicated that the EOs of both plants exerted substantial bactericidal potency, with MIC values greater than 7 mg/mL across all tested microorganisms. Interestingly, P. aeruginosa (ATCC 27852) remained unaffected by any of the EOs.
These findings are consistent with previously reported results for Prangos and Cachrys species (5, 13, 14). For instance, Rahman et al. (12) reported that the petroleum ether extract of P. platychlaena leaves exhibited strong bactericidal activity against S. aureus and P. aeruginosa, with MIC values of 0.23 and 1.33 mg/mL, respectively — surpassing those of other extracts evaluated in their study.
A previous report from Iraq by Rahman and Safar (3) indicated that different concentrations (5.00 to 10.00 mg/mL) of the petroleum ether extract obtained from the leaves and flowers of C. scabra exhibited significant bactericidal activity against all tested strains. In contrast, the EOs and ethanol extracts showed weak antibacterial activity against P. aeruginosa, even at the highest tested concentrations. However, Rahman et al. (11) reported that the EOs extracted from the flowers and leaves of P. platychlaena, which are rich in terpenes, demonstrated strong bactericidal potency against S. aureus and P. aeruginosa, with MIC values of 0.86 ± 0.13 and 1.43 ± 0.06 mg/mL for the flower EO, and 2.54 ± 0.32 and 1.16 ± 0.08 mg/mL for the leaf EO, respectively.
Conversely, Uzel et al. (15) from Turkey reported that P. platychlaena EO, with MIC values reaching up to 144 mg/mL, exhibited weak bactericidal efficacy against S. aureus, E. faecalis, B.subtilis, E. coli, P. aeruginosa, and Salmonella typhimurium, compared to P. uechtritzii EO.
4.4. Conclusions
Plant-derived natural products, particularly EOs, are increasingly valued in the pharmaceutical, food, cosmetic, and perfumery industries. Their extensive use as antioxidants and antimicrobials highlights their potential as effective herbal medicinal alternatives. In this study, the EOs of P. platychlaena and C. scabra exhibited significant antioxidant potential, as demonstrated by both DPPH and ABTS assays. Additionally, these EOs showed considerable antibacterial activity against all tested microorganisms, except for P. aeruginosa, which was resistant to their effects.
However, further studies are warranted to comprehensively evaluate the in vitro and in vivo biological activities of these EOs, particularly concerning their safety, toxicity, and potential mechanisms of action.