1. Introduction
For decades, breast cancer (BC) has been the most prevalent cancer worldwide, with approximately 2.26 million new cases diagnosed annually (1). Recently, cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) have become a focal point in BC research (2). Researchers have developed a series of CDK4/6i, including palbociclib, ribociclib, and abemaciclib (3). Among BC subtypes, hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) tumors are the most prevalent, accounting for approximately 70% of all cases (4).
Cyclin-dependent kinase 4/6 inhibitors, namely palbociclib, ribociclib, and abemaciclib, when combined with endocrine therapy (ET), have been shown to double median progression-free survival (PFS) compared with single-agent ET in both first-line and later-line settings in pivotal phase 3 trials. Additionally, they improved median overall survival (OS) in some cases (5, 6). When combined with fulvestrant, these three drugs also significantly improved PFS in patients whose tumors progressed during prior ET (7-9).
Palbociclib is a first-in-class, potent, highly selective, orally administered, reversible cyclin-dependent kinase 4/6 inhibitor (10). Preclinical studies revealed that palbociclib combined with ET preferentially and synergistically inhibits the cell cycle in human estrogen receptor–positive BC cell lines (11). However, outcomes may vary depending on population, as some studies included patients of Asian ethnicity, whose dietary habits and medical environments differ from Western countries, potentially influencing clinical outcomes (12, 13). Here, we report PFS and OS data evaluating the efficacy of palbociclib combined with ET compared with placebo plus ET or ET alone in patients with advanced BC.
2. Materials and Methods
2.1. Study Design and Protocol
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for PRISMA guidelines (14). The study protocol was designed based on the Population, Intervention, Comparison, and Outcomes (PICO) framework. PICO question: In patients with HR+ and HER2- or metastatic BC (Population), does the combination of palbociclib plus ET (Intervention), compared with ET alone or placebo plus ET (Comparison), improve survival outcomes such as PFS and OS (Outcome)?
2.2. Search Strategy
A comprehensive literature search was conducted in PubMed, Scopus, and Web of Science databases from inception to November 9, 2025, without any language or date restrictions. The following search terms were used: ("breast cancer" or "breast neoplasm*" or "breast carcinoma" or "mammary carcinoma") and ("palbociclib" or "Ibrance" or "CDK4/6 inhibitor" or "cyclin-dependent kinase 4/6 inhibitor") and ("randomized controlled trial" or "randomised controlled trial" or "randomized trial" or "randomised trial" or "randomized study" or "randomised study"). Additionally, a manual search was performed in Google Scholar (first ten pages of results) and the reference lists of relevant reviews and original studies to identify any additional eligible trials.
2.3. Eligibility Criteria
Studies were included if they met the following criteria: (A) Randomized controlled trial (RCT). (B) Adult patients with HR+/HER2- advanced or metastatic BC. (C) Intervention group was palbociclib combined with ET (e.g., letrozole or fulvestrant). (D) Control group was ET alone or placebo plus ET. (E) Studies reported at least one survival endpoint — PFS or OS.
2.4. Data Extraction and Quality Assessment
Two investigators independently extracted data on study characteristics, patient demographics, interventions, and outcomes. Discrepancies were resolved by discussion or consultation with a third reviewer. The Cochrane Collaboration’s Risk of Bias Tool was used to evaluate seven domains.
2.5. Statistical Analysis
Hazard ratios (HRs) and 95% confidence intervals (CIs) for PFS and OS were extracted from each trial. A fixed-effect model (Mantel–Haenszel method) was applied to calculate pooled estimates, accounting for potential between-study heterogeneity. Heterogeneity was assessed using the I² statistic, with I² > 50% indicating substantial heterogeneity. Publication bias was evaluated visually by funnel plots and statistically using Egger’s and Begg's tests, with a P < 0.10 indicating significant bias. All analyses were performed using Comprehensive Meta-Analysis (version 3.0) software.
3. Results
3.1. Study Selection
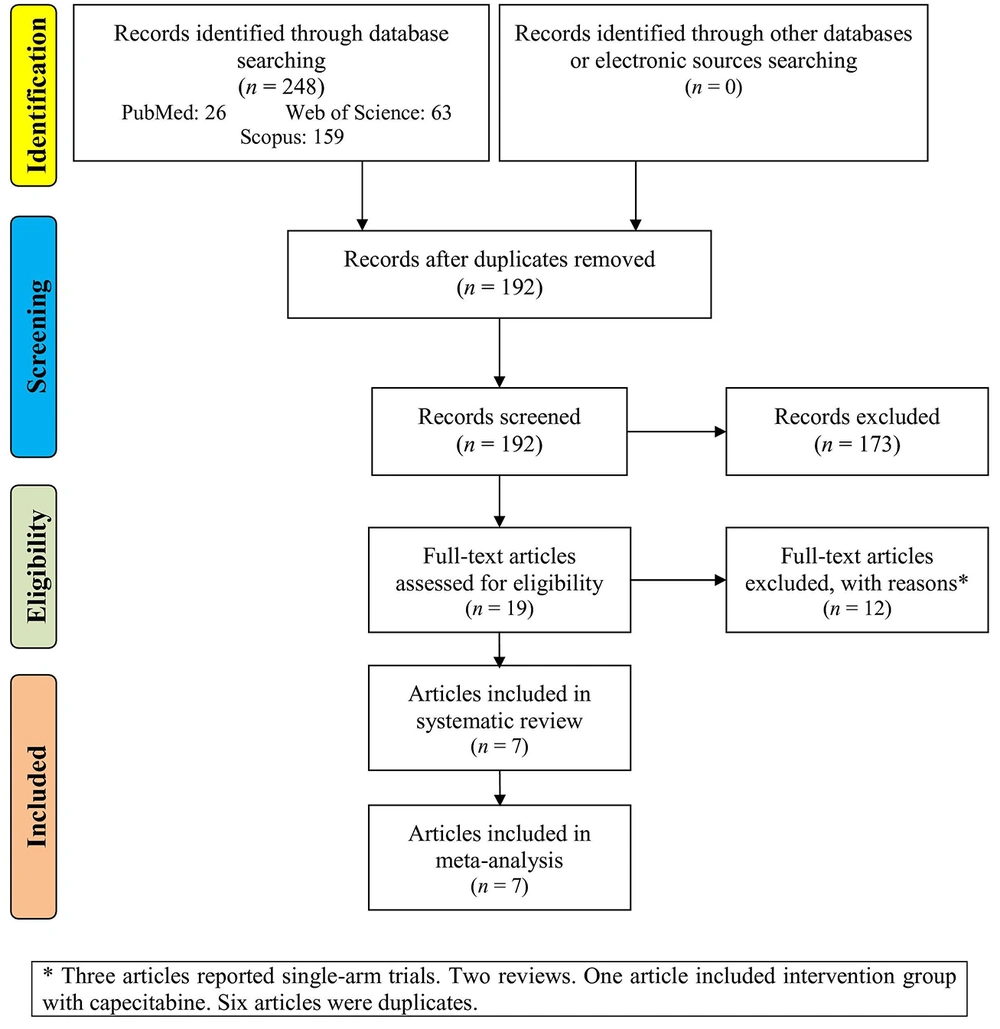
A total of 248 records were identified through database searches, including 26 from PubMed, 63 from Web of Science, and 159 from Scopus, with no additional records obtained from other sources. After removing duplicates, 192 records remained for screening. Of these, 173 were excluded based on title and abstract review, leaving 19 full-text articles assessed for eligibility. Twelve of these were excluded for specific reasons, including three single-arm trials, two review articles, one study involving an intervention group with capecitabine, and six duplicate records. Ultimately, seven articles (7, 15-20) met the inclusion criteria and were incorporated into both the systematic review and the meta-analysis (Figure 1).
3.2. Characteristics of Cases
The seven studies included in the meta-analysis evaluated the efficacy of palbociclib combined with ET (letrozole or fulvestrant) in patients with BC (Table 1). These trials, conducted between 2015 and 2024, were large-scale, randomized controlled trials registered in recognized clinical trial databases.
| The First Study, Publication Year | RegistryNumber | Intervention Group (N) | Control Group (N) | Outcome | Follow-up Time (Months) |
|---|---|---|---|---|---|
| Finn, 2020 (15) | NCT00721409 | Palbociclib + Letrozole (84) | Letrozole (81) | OS | 84 |
| Cristofanilli, 2022 (16) | NCT01942135 | Palbociclib + Fulvestrant (347) | Placebo + Fulvestrant (174) | OS | 84 |
| Slamon, 2024 (17) | NCT01740427 | Palbociclib + Letrozole (444) | Placebo + Letrozole (222) | OS | 108 |
| Mukai, 2019 (18) | NCT01740427 | Palbociclib + Letrozole (32) | Placebo + Letrozole (14) | PFS | 33 |
| Albanell, 2022 (19) | NCT02690480 | Palbociclib + Fulvestrant (265) | Placebo + Fulvestrant (130) | PFS | 36 |
| Cristofanilli, 2016 (7) | NCT01942135 | Palbociclib + Fulvestrant (265) | Placebo + Fulvestrant (130) | PFS | 15 |
| Turner, 2015 (20) | NCT01942135 | Palbociclib + Fulvestrant (347) | Placebo + Fulvestrant (174) | PFS | 12 |
Abbreviations: OS, overall survival; PFS, progression-free; N, number of cases.
Among them, three studies reported OS outcomes, while four studies focused on PFS. The sample sizes across intervention groups ranged from 32 to 444 patients, with control groups including 14 to 222 patients. The follow-up duration varied substantially, from 12 months to 108 months, indicating a mix of short- and long-term data across studies.
3.3. Pooled Analyses
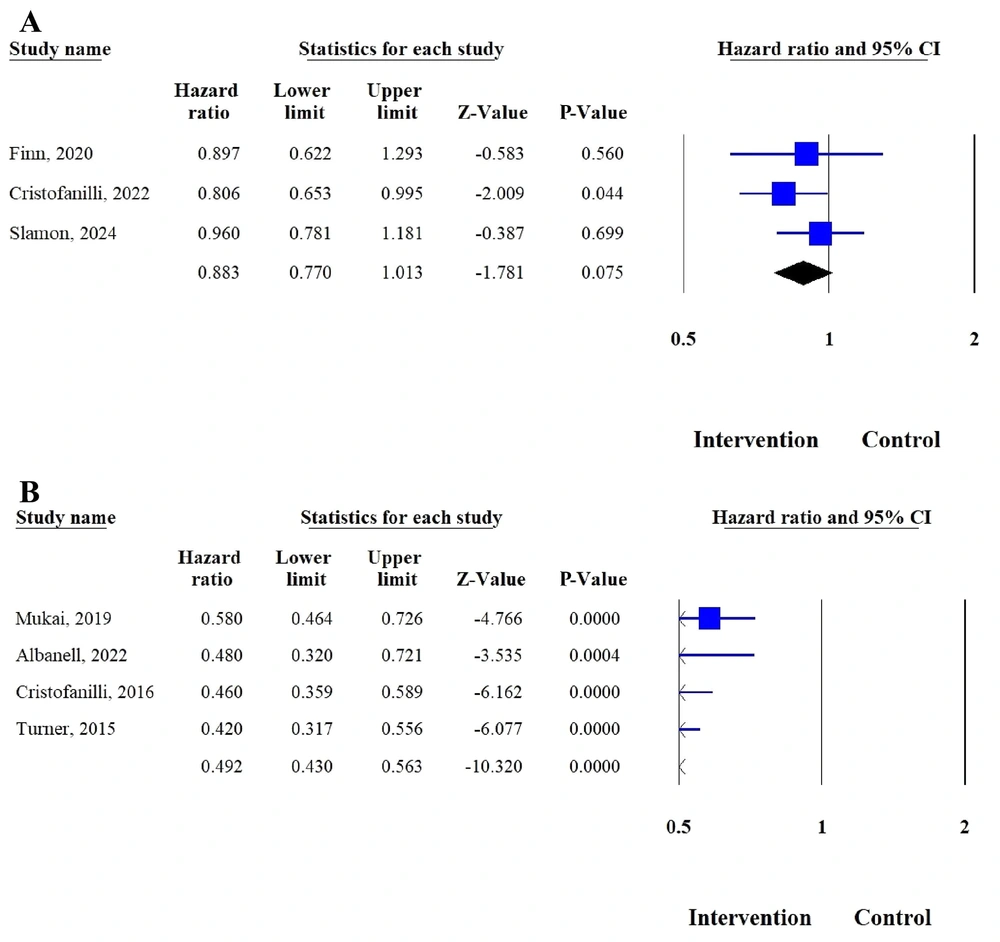
Figure 2A presents a forest plot summarizing the HRs for OS comparing the intervention group (palbociclib plus ET) with the control group (ET alone or with placebo) across three studies. The pooled analysis yielded a pooled HR of 0.883 (95% CI: 0.770 - 1.013, P = 0.075), indicating a trend toward improved survival in the intervention group, though this result did not reach statistical significance. Overall, these findings suggest that palbociclib in combination with ET may modestly enhance OS in patients with hormone receptor-positive BC, but further evidence is required to confirm a definitive survival benefit.
Figure 2B illustrates a forest plot showing the HRs for PFS comparing the intervention group (palbociclib plus ET) with the control group (ET alone or with placebo) across four studies. The pooled analysis demonstrated a combined HR of 0.492 (95% CI: 0.430 - 0.563, P < 0.001), indicating a significant 48% reduction in the risk of disease progression or death with palbociclib-based treatment. All studies consistently favored the intervention group, with CIs that did not cross the line of no effect. These findings strongly support that palbociclib in combination with ET provides a substantial and statistically significant improvement in PFS compared with ET alone in patients with hormone receptor-positive BC.
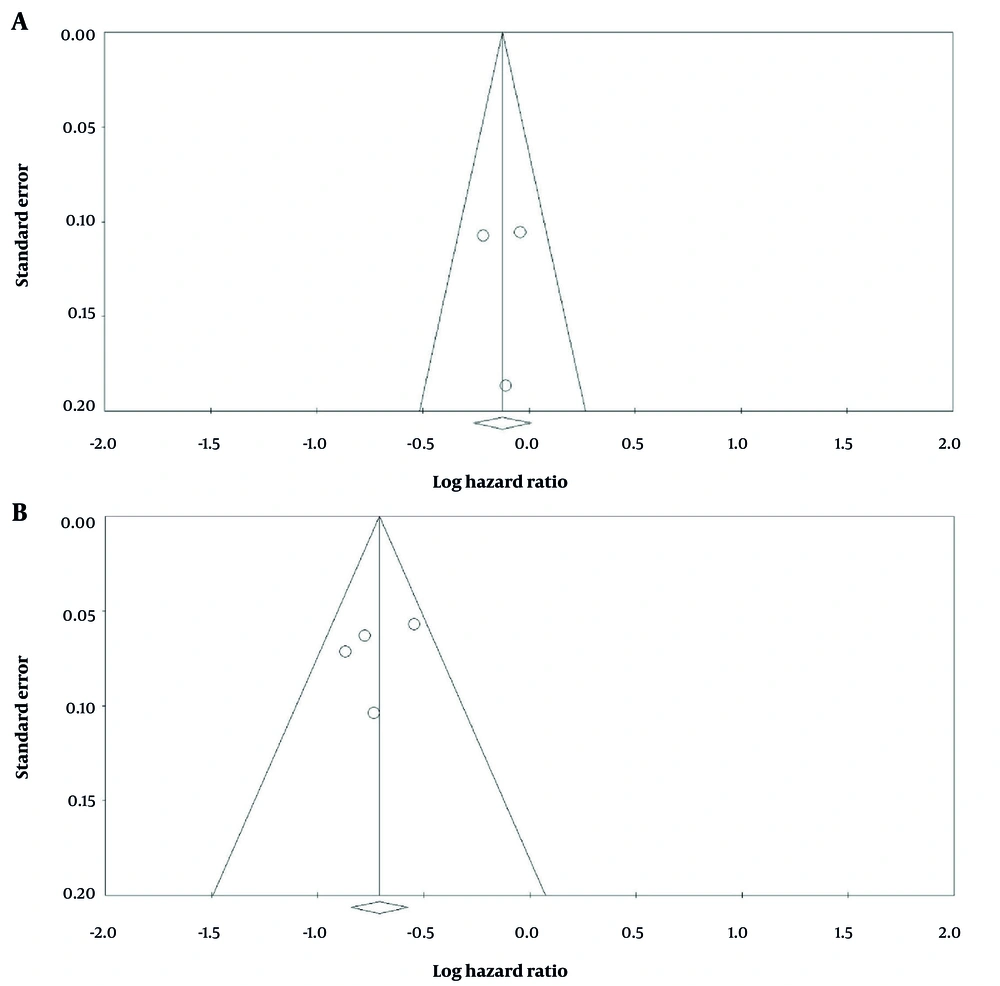
Figure 3A presents a funnel plot evaluating potential publication bias for the OS analysis comparing the intervention group (palbociclib plus ET) with the control group. The plot shows a symmetric distribution of studies around the pooled effect size, suggesting the absence of substantial publication bias. Statistical assessment using Begg’s test (P = 0.601) and Egger’s test (P = 0.968) further confirmed this finding, as both P-values were greater than 0.10. Therefore, there was no evidence of significant publication bias among the included studies assessing OS.
Figure 3B shows a funnel plot of the HR for PFS comparing the intervention group with the control group. Begg's test yielded a P-value of 0.796, and Egger's test yielded a P-value of 0.576. Since both P-values are greater than 0.10, there is no evidence of significant publication bias.
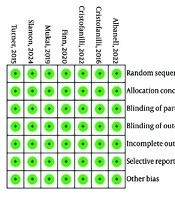
Figure 4 presents a comprehensive assessment of bias risk across the included clinical studies. Section A, the risk of bias summary, shows that 100% of the studies evaluated exhibit a low risk of bias across all seven domains. This is visually represented by fully green bars, indicating methodological consistency and rigor. Section B, the risk of bias graph, reinforces these findings by displaying a matrix in which each study shows a low risk of bias in every domain, marked by green circles with plus signs. Together, these visuals confirm that the studies included in the analysis are of high quality and free from significant methodological flaws, enhancing the reliability and validity of the overall evidence base.
4. Discussion
Seven randomized controlled trials were selected from an initial pool of 248 records to evaluate the efficacy of palbociclib combined with ET in hormone receptor-positive BC. These studies, conducted between 2015 and 2024, varied in sample size and follow-up duration, and reported either OS or PFS outcomes. Pooled analysis showed a non-significant trend toward improved OS, but a significant benefit in PFS, indicating a 48% reduction in disease progression or death. No substantial publication bias was detected, and all studies demonstrated a uniformly low risk of bias across key methodological domains, confirming the reliability of the evidence.
The results reinforce the key observation that endocrine monotherapy shows limited efficacy in patients whose disease has progressed despite prior endocrine sensitivity, highlighting the need for routine use of more effective combination therapies (7, 20-22). The combination of hormone therapy and palbociclib has been shown to significantly enhance treatment outcomes in patients, leading to higher objective response rates, increased clinical benefit, and prolonged PFS compared with hormone therapy alone (7, 18, 19). Subgroup analyses of patients from various Asian countries, including Korea and Japan, further support the efficacy of palbociclib in Asian patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced BC, demonstrating consistent improvements in PFS and clinical response across these populations (18, 23, 24).
Recent evidence from two systematic reviews and meta-analyses strongly supports the superiority of combining CDK4/6i with ET over ET alone, demonstrating enhanced efficacy in terms of objective response, clinical benefit, and PFS for the vast majority of patients (4, 25). This synergy underscores the value of cyclin-dependent kinase 4/6 inhibition in overcoming resistance and improving disease control in endocrine-sensitive BC.
Limitations: (1) Limited number of trials and OS data – Only seven randomized controlled trials were included, and just three reported OS outcomes, which reduces statistical power and limits conclusions about long-term survival benefits. (2) Variability in study design and follow-up – The included trials differed in sample sizes, interventions (letrozole versus fulvestrant), and follow-up durations (12-108 months) that may affect pooled estimates. (3) Restricted subgroup and real-world analysis – The meta-analysis did not explore patient subgroups (e.g., age, prior treatments, menopausal status) or real-world outcomes, limiting the ability to identify which populations benefit most from palbociclib.
5. Conclusions
This systematic review and meta-analysis of seven randomized controlled trials demonstrated that palbociclib in combination with ET provides a significant improvement in PFS (HR = 0.492, P < 0.001) in patients with hormone receptor-positive BC, while showing a non-significant trend toward improved OS (HR = 0.883, P = 0.075). The included studies were of high methodological quality, with uniformly low risk of bias and no evidence of publication bias, supporting the robustness of the findings.
The results highlight the clinical value of palbociclib as an effective addition to ET, particularly in delaying disease progression. This benefit translates into meaningful improvements in patient management, offering extended disease control and potentially better quality of life. Although the OS advantage did not reach statistical significance, the consistent PFS benefit underscores palbociclib’s role as a standard therapeutic option in hormone receptor-positive BC.
Further long-term studies and pooled analyses with larger patient populations are needed to clarify the impact of palbociclib on OS and to identify subgroups that may derive the greatest benefit. Future research should also explore biomarkers predictive of response, cost-effectiveness analyses, and real-world outcomes to optimize patient selection and treatment strategies.