1. Introduction
Bicuspid aortic valve (BAV) is the most common congenital cardiac anomaly, with a prevalence of 1 - 2% and a clear male predominance (1, 2). Combining a durable aortic valve repair with a minimally invasive approach can therefore be appealing both for patients and for surgeons. To the best of our knowledge, based on the available literature, there have been no previous reports of aortic valve repair using the HAART 200 ring via a right anterior thoracotomy (RAT) approach (3-6). We aim to share our experience with this novel combination and discuss a small technical modification that may facilitate its safe and reproducible application.
2. Case Presentation
A 20-year-old man (body surface area: 2.0 m²) presented with early fatigue and mild exertional dyspnea. He had a known history of BAV but was otherwise healthy. Preoperative transthoracic and transesophageal echocardiography (TEE) revealed severe aortic regurgitation with no aortic stenosis (AS), reverse doming of the anterior mitral valve leaflet due to severe eccentric aortic insufficiency (AI), a left ventricular end-diastolic diameter (LVEDD) of 67 mm, and preserved left ventricular ejection fraction (LVEF: 50 - 55%). Computed tomography angiography demonstrated an aortic annulus of 28 mm, sinus of Valsalva 37 mm, and ascending aorta 39 mm, with normal coronary arteries.
Given his age and preference, anatomy, and favorable leaflet quality, aortic valve (AV) repair using intra-annular HAART ring with RAT approach was planned.
2.1. Surgical Technique
After cardiac monitoring was established, 50 micrograms of fentanyl and 1 mg of midazolam were administered, and local lidocaine was injected at the left radial site before placement of the arterial catheter. Subsequently, after pre-oxygenation, 300 micrograms of fentanyl, 2 mg of midazolam, 80 mg of lidocaine, 20 mg of etomidate, and 20 mg of cisatracurium were administered for induction of anesthesia, followed by endotracheal intubation with a 7.5-mm tube.
Two central venous lines were inserted via the right and left internal jugular veins. Sevoflurane was used for maintenance of anesthesia. Then, in the supine position, following systemic heparinization with a full dose, peripheral femoral–femoral cannulation was performed under intraoperative transesophageal echocardiographic (IOTEE) guidance. A right anterior mini-thoracotomy was made through the third intercostal space. After establishing cardiopulmonary bypass (CPB), pericardiotomy was performed, and a vent was inserted through the right upper pulmonary vein. The aorta was cross-clamped using a Chitwood clamp, and cardioplegic arrest was achieved with del Nido solution.
Following aortotomy and placement of traction sutures, excellent exposure of the aortic valve was obtained. The valve was a Type I BAV with good leaflet quality and no calcification. The fibrotic raphe was carefully excised using sharp dissection, and the tethered fused leaflet was detached and released from the aortic annulus.
Using the dedicated HAART 200 sizing system and based on intraoperative and echocardiographic findings, a ring size 23 was selected.
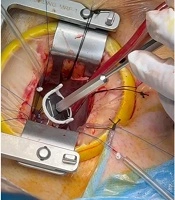
Fixation sutures were first placed with double-armed 3 - 0 Ethibond pledgeted stitches at the appropriate subcommissural areas of both commissures. The sutures were then passed through the ring posts of the HAART device, through the second pledgets and the adjacent annulus, and the ends were snared (Figure 1).
The HAART ring was positioned precisely beneath the aortic valve, ensuring that its posts had no direct contact with the leaflets or commissures. For better visualization and maneuverability in the limited field, the snare was slightly loosened, releasing the ring by about 5 mm. Three looping sutures for each leaflet were then placed using double-armed 4 - 0 Ethibond sutures with the special pledgets provided in the HAART ring package, securing the ring just at the ventricular surface of the leaflets in an intraannular fashion.
The snare was then tightened again, and all eight sutures were tied using 4 - 0 Ethibond with eight secure knots. The suture ends were trimmed close to the last knot to avoid lateralization of the fixing sutures, which is usually time-consuming.
A 6 - 0 Prolene suture was used to repair the leaflet prolapse and the residual gap in the previously fused leaflet, completing the valve repair. A saline test demonstrated excellent valve competence. The aortotomy was closed, the vent removed, and controlled root reperfusion was performed via the cardioplegia cannula in the ascending aorta. Adequate root pressure confirmed valve competence. After administration of lidocaine, the aortic cross-clamp was removed, and the patient was weaned easily from cardiopulmonary bypass using a low dose of norepinephrine.
2.2. Postoperative Course
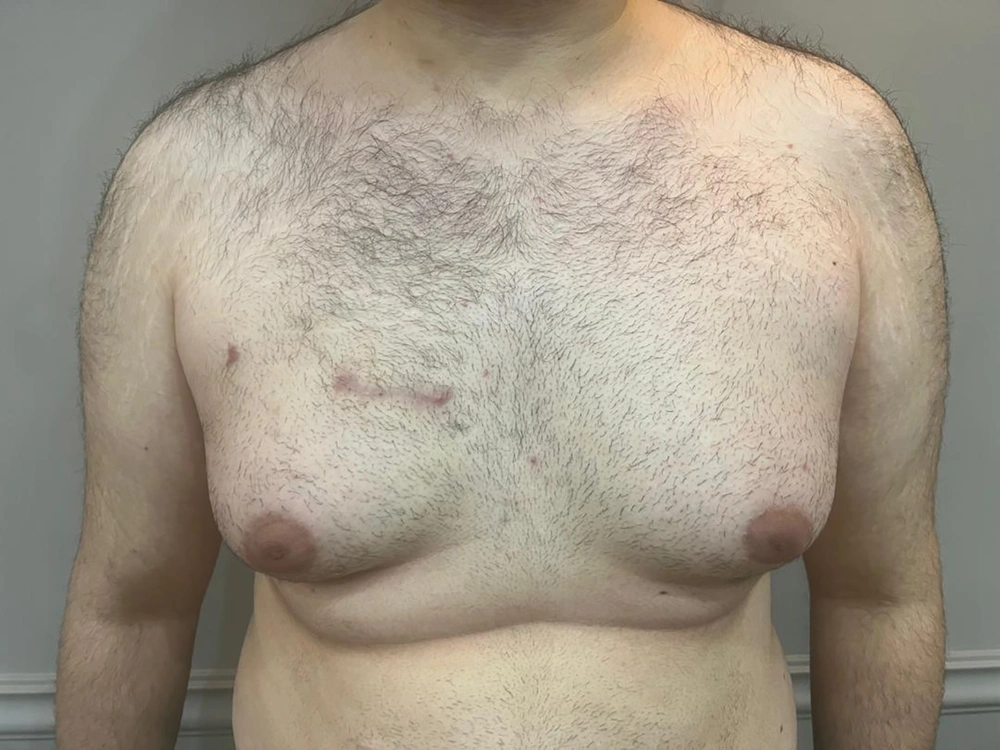
Echocardiographic assessment by both intraoperative TEE and in the immediate post-operative transthoracic echocardiography (TTE) demonstrated a successful valve result with only trivial aortic regurgitation and no stenosis. Left ventricular systolic function was mildly reduced at 45%. Total postoperative drainage was 350 ml. The patient was extubated early and had an uneventful recovery. He was discharged in good condition on a low dose of Apixaban for 8 weeks. During early follow-up, he was in good functional class with trivial AI and no complication. Follow-up echocardiography at three months post aortic valve repair showed a satisfactorily sized left ventricle (EDD 5.2 cm, ESD 3.4 cm) with preserved systolic function (LVEF 50%) and mild AI. Figure 2 shows the appearance of the RAT incision after three months.
3. Discussion
The BAV is the most common congenital cardiac anomaly. Most patients become symptomatic in the third or fourth decade of life, requiring surgical intervention. Mechanical valve replacement in this age group is associated with long-term anticoagulation challenges and lifestyle limitations, while biological valves, though avoiding anticoagulation, are prone to early structural degeneration and reoperation. The Ross procedure, despite its physiological benefits, remains technically demanding and carries potential long-term complications due to homograft degeneration in the pulmonary position. Although repair of BAVs by experienced surgeons has yielded promising short-term results, durable long-term outcomes remain limited (1). One of the main challenges in aortic valve repair — particularly in patients with large aortic annuli — is the reconstruction and stabilization of the annulus (3, 4). Annular dilatation and lack of geometric stabilization are major contributors to failed repair, residual regurgitation, and recurrence of AI in the early and midterm periods (5, 6). Minimally invasive cardiac surgery through small incisions offers reduced postoperative pain, bleeding, and infection rates, as well as shorter hospital stay, faster recovery, and greater patient satisfaction (7).
This early experience suggests that the combination of a HAART ring and a RAT approach can be a safe and reproducible alternative for selected young patients with bicuspid aortic regurgitation and suitable leaflet morphology. Combining this technology with a mini-thoracotomy approach potentially reduces surgical trauma, transfusion, hospitalization, faster recovery, and improves patient quality of life.
3.1. Conclusions
HAART ring-assisted aortic valve repair via a RAT approach is feasible, safe, and effective in selected patients with BAV regurgitation. This technique may broaden the application of minimally invasive valve repair strategies and represents a promising evolution in the treatment of young patients with isolated AI.