1. Background
Osteoporosis is characterized by reduced bone mass and microarchitecture (1). Over the past century, the global incidence of osteoporosis has significantly increased, primarily driven by the extended average lifespan — attributed to enhanced safety measures, improved healthcare, adherence to public health guidelines, and the consequent growth of the elderly population (2, 3). This trend is further compounded by an escalating economic burden associated with fracture-related healthcare costs (4, 5).
Osteoporosis is defined through the assessment of bone mineral density (BMD). As per the World Health Organization (WHO) criteria, osteoporosis is characterized by a BMD that is 2.5 standard deviations or more below the average BMD of young, healthy women, indicated by a t-score of less than -2.5 SD. Dual-energy X-ray absorptiometry (DXA) is the most widely accepted method to measure BMD. Diagnostic criteria based on the t-score for BMD serve as the recommended criterion for developing pharmaceutical interventions in osteoporosis (5).
Osteoporosis is categorized into primary (including type I and type II) and secondary forms. Primary osteoporosis predominantly affects post-menopausal women and individuals of both genders aged over 70, primarily due to the natural aging process. Secondary osteoporosis, meanwhile, results from various factors including diseases, treatments, or idiopathic causes. Among the contributing factors are systemic diseases, endocrine disorders, malignant neoplasms, chronic use of glucocorticoids, lifestyle conditions, habits, as well as major depression (6-8).
The prevalence of osteoporosis was found to be twice as high in individuals with an estimated glomerular filtration rate (eGFR) less than 60 mL/min compared to those with eGFR greater than 60 mL/min in a national study (9). In a prospective cohort study involving disabled individuals with CKD, it was observed that CKD was associated with a moderate increase in fracture risk, even after adjusting for race, BMD, and age (10). Fractures can further lead to increased mortality rates among CKD patients, both with and without dialysis (11, 12).
Screening for fracture risk often involves assessing clinical risk factors combined with DXA to measure BMD. However, despite the availability of screening guidelines and the known complications of osteoporotic fractures, the diagnosis of osteoporosis is frequently overlooked, resulting in a low screening rate for this preventable disease (2). While DXA is the standard method for diagnosing osteoporosis and monitoring treatment through BMD measurement in the spine and pelvis, the WHO has emphasized the need for considering additional factors, beyond the t-score calculated using DXA, to identify individuals at a higher risk of fractures. The calculation of absolute fracture probability based on clinical risk factors is now recognized as crucial (13).
The DXA has some recognized limitations. Its planar projection makes it sensitive to factors such as vertebral size, degenerative joint disease, and patient positioning. Moreover, doubts exist regarding whether DXA, as a dual-energy method, can accurately measure the true bone mineral content, given the individual and non-uniform composition of bone mineral and fat/soft tissue in the human body. To ensure consistency and independent monitoring, calibration between devices and facilities is crucial for comparative analysis of results. Furthermore, skilled technician training is essential for obtaining reliable DXA measurements (14).
While alternative techniques like quantitative computed tomography (QCT) are emerging for assessing BMD and bone quality, they have yet to replace or complement DXA in diagnosing osteoporosis and evaluating fracture risk. Studies focusing on the spine have shown that QCT captures age-related trabecular bone loss more accurately than DXA. Additionally, QCT exhibits higher sensitivity in differentiating vertebral fractures among postmenopausal women (15).
The widespread use of computed tomography (CT) scans in clinical evaluations presents another reason for considering imaging techniques in osteoporosis diagnosis. In 2015, 29 member countries of the Organization for Economic Cooperation and Development (OECD) reported an average of more than 140 CT scans per 1,000 individuals. Given the likely inclusion of vertebrae in many of these scans, the potential for osteoporosis screening by analyzing bone quality parameters in CT data is enormous. Although studies have explored this concept in recent years, it remains unclear if and when opportunistic screening methods utilizing CT scans will be adopted for osteoporosis diagnosis. Notably, advancements in CT acquisition and image reconstruction methods, with substantially reduced radiation doses, may hold promise for assessing vertebral fracture risk (16).
Given the frequent use of chest or abdominal CT scans for other clinical purposes, these imaging studies offer a valuable opportunity to enhance osteoporosis screening rates without incurring additional costs, time, or radiation exposure for patients. By utilizing sagittal reconstruction of existing CT data, healthcare providers can efficiently detect incidental findings such as vertebral compression fractures (17).
2. Objectives
The present study aimed to compare the diagnostic efficacy of CT scans and bone densitometry in identifying osteoporosis and osteopenia among patients with chronic kidney failure. Focusing on individuals referred to Baqiyatallah Hospital in 2021, the research investigated the correlation between L1 vertebral bone density (via CT) and standard BMD measurements, alongside clinical factors influencing bone health in this population. The findings underscore that integrating CT-based opportunistic screening into routine care could serve as a pivotal strategy for early detection and prevention of osteoporosis and osteopenia in CKD patients, thereby improving clinical outcomes.
3. Methods
3.1. Study Population and Design
The study enrolled patients with kidney failure who were admitted to Baqiyatallah Hospital in the year 2021 due to COVID-19 and had undergone a lung CT scan. Of the 958 patients initially screened for eligibility, 322 met the inclusion criteria after exclusions for: (1) Incomplete medical records, (2) time interval greater than 6 months between CT and DXA, and (3) absence of CKD (as per exclusion criteria). Based on the given formula, with α = 5%, a sensitivity of 0.9, an estimation error of 5%, and a prevalence rate of osteopenia and osteoporosis of 0.6 among patients with kidney failure in this study, ultimately, 322 individuals were included in the analysis.
3.2. Procedure
After receiving ethical approval for the study, a list of patients who had undergone bone density measurement for rheumatological indications was extracted. Subsequently, a list of patients admitted to Baqiyatallah Hospital due to COVID-19 and who had undergone a lung CT scan was obtained from the Baqiyatallah Hospital electronic archives. A maximum interval of 6 months between bone density measurement and CT scan was considered. The study included data from 958 men and women aged 18 to 100 years who had been referred to Baqiyatallah Hospital for ruling out or confirming COVID-19 infection and had undergone a chest CT scan. These individuals had also undergone DXA for measuring bone density either 6 months before or after the CT scan. The available CT scan images were two-dimensional reconstructions in coronal and sagittal sections, along with bone density measurement reports. Hounsfield units (HU) measurements were obtained by an experienced radiologist using Picture Archiving and Communication System (PACS) software. Additionally, the modification of diet in renal disease (MDRD) number was calculated to determine the different stages of kidney failure for each patient, based on average creatinine levels during hospitalization and the recorded age and gender from the patient's medical records. Glomerular filtration rate (GFR) in mL/min per 1.73 m2 was calculated using the following formula: GFR = 175 × SerumCr-1.154 × age-0.203 × 1.212 (if patient is black) × 0.742 (if female).
The HU measurement for each vertebra was obtained by selecting the largest region of interest (ROI) in the mid-air part of the vertebra, without including the cortical margin. We focused on the L1 vertebra due to: (1) Its consistent inclusion in routine chest/abdominal CT scans (unlike lower lumbar vertebrae, which may be omitted in limited scans); (2) lower susceptibility to degenerative changes compared to L2 - L4, as supported by prior studies (14, 17); and (3) clinical feasibility for opportunistic screening. The software calculated the average HU value within the ROI. Typically, one or three measurements were obtained from this region.
The main objective of the study was to compare the standard method of measuring bone density with incidental bone findings from the lung CT scan in a wide age range of patients undergoing the scan for diagnosis or further treatment. Additionally, the associations between these bone findings and various factors mentioned earlier were investigated. A researcher-developed checklist was used, incorporating the defined study variables such as age, gender, stage of kidney failure (GFR), kidney function (MDRD), Hounsfield number of the first lumbar vertebra, bone density, and the presence of underlying diseases (diabetes and blood pressure).
3.3. Inclusion and Exclusion Criteria
The inclusion criteria comprised patients aged ≥ 18 years who underwent BMD measurement during the study period (2021) within a 6-month interval, aimed at confirming or ruling out COVID-19 infection at Baqiyatallah Hospital, and had documented stages of chronic kidney failure. Patients were excluded from the study if they exhibited any abnormalities in the T12-L2 spine detectable on CT scan, such as spinal tumors, spondylopathy (ankylosing spondylitis), infectious spondylitis, diffuse idiopathic skeletal hyperostosis, presence of lumbar prostheses, prior vertebral fractures, metastatic bone conditions, or trauma-related bone damage. Healthy individuals in terms of kidney function, those with missing medical records, and patients with a time interval of more than 6 months between BMD and CT scan were also excluded from the study.
3.4. Definition of Terms
- Osteoporosis: A condition characterized by a bone density decrease of 2.5 standard deviations or more below the average of healthy young adults of the same gender and race, denoted as t-score ≤ -2.5.
- Osteopenia: A condition characterized by a bone density decrease of 1 standard deviation below the average of healthy young adults of the same gender and race, denoted as 1 ≤ t-score < 2.5.
- Osteoporosis (based on Hounsfield measurement of the first lumbar vertebra): A HU value less than 110.
- Osteopenia (based on Hounsfield measurement of the first lumbar vertebra): A HU value less than 135 but greater than or equal to 110.
- The HU: A dimensionless unit used in CT scanning to express the CT number in a standardized manner.
- Chronic renal failure: A condition encompassing various pathophysiological processes associated with abnormal kidney function and a progressive decline in GFR.
- Sensitivity: The proportion of truly sick individuals who are correctly classified as part of the patient group or the proportion of individuals who are truly sick and have a positive screening test result [sensitivity = a/(a + c)].
- Specificity: The proportion of truly healthy individuals who are correctly classified as part of the healthy group or the proportion of individuals who are truly healthy and have a negative screening test result [specificity = d/(b + d)].
- Positive predictive value: The proportion of individuals who are truly sick and have a positive test result [positive predictive value = a/(a + b)].
- Negative predictive value: The proportion of individuals who are truly healthy and have a negative test result [negative predictive value = d/(c + d)].
- Accuracy of the test: The ratio of correctly identified cases to the total number of samples, calculated as (a + d)/n.
3.5. Statistical Analysis
The collected data were entered into SPSS 24.0 software, and the relationship between age, sex, underlying diseases (diabetes and blood pressure), and osteoporosis and osteopenia in patients with renal failure was analyzed. The data analysis involved summarizing and describing categorical variables using frequencies and percentages, and quantitative variables using means and standard deviations. Logistic regression tests, as well as Pearson and Spearman correlation coefficients, were performed using SPSS software. Sensitivity, specificity, positive predictive value, and negative predictive value were calculated using relevant formulas. The significance level was set at P < 0.05.
4. Results
A total of 322 patients were included in the study, among whom 268 (83.2%) were female and 54 (16.8%) were male. Table 1 presents the relationship between gender and the prevalence of osteoporosis and osteopenia based on t-scores of the spine, femur, forearm, and L1 density.
| Variables | BMD Status | Total | Chi-square Test (P) | ||
|---|---|---|---|---|---|
| Normal | Osteopenia | Osteoporosis | |||
| Relationship between gender and osteoporosis and osteopenia based on t-score of the spine | 0.864 | ||||
| Male | 26 (48.1) | 19 (35.2) | 9 (16.7) | 54 (100) | |
| Female | 123 (46.1) | 104 (39) | 40 (15) | 267 (100) | |
| Total | 149 (46.4) | 123 (38.3) | 49 (15.3) | 321 (100) | |
| Gender relationship with osteoporosis and osteopenia based on femur t-score | 0.899 | ||||
| Male | 23 (42.6) | 28 (51.9) | 3 (5.6) | 54 (100) | |
| Female | 118 (44.4) | 130 (48.9) | 18 (6.8) | 266 (100) | |
| Total | 141 (44.1) | 158 (49.4) | 21 (6.6) | 320 (100) | |
| Relationship between gender and osteoporosis and osteopenia based on t-score | 0.164 | ||||
| Male | 1 (7.1) | 7 (50) | 6 (42.9) | 14 (100) | |
| Female | 24 (32) | 28 (37.3) | 23 (30.7) | 75 (100) | |
| Total | 25 (28.1) | 35 (39.3) | 29 (32.6) | 89 (100) | |
| Gender relationship with osteoporosis and osteopenia based on L1 density | 0.557 | ||||
| Male | 31 (57.4) | 18 (33.3) | 5 (9.3) | 54 (100) | |
| Female | 165 (61.6) | 71 (26.5) | 32 (11.9) | 268 (100) | |
| Total | 196 (60.9) | 89 (27.6) | 37 (11.5) | 322 (100) | |
Abbreviation: BMD, bone mineral density.
a Values are expressed as No. (%).
The majority of patients (53.7%) fell within the age group of 40 - 60 years, followed by 38.82% in the group aged 60 years and older (125 individuals), and 7.45% (24 individuals) in the group younger than 40 years. Table 2 presents the relationship between age and the prevalence of osteoporosis and osteopenia based on t-scores of the spine, femur, forearm, and L1 density.
| Variables | BMD Status | Total | Chi-square Test (P) | ||
|---|---|---|---|---|---|
| Normal | Osteopenia | Osteoporosis | |||
| The relationship between age distribution of patients with osteoporosis and osteopenia based on spine t-score | 0.0 | ||||
| Less than 40 | 14 (58.3) | 8 (33.3) | 2 (8.3) | 24 (100) | |
| 40 - 60 | 92 (53.5) | 65 (37.8) | 15 (8.7) | 172 (100) | |
| More than 60 | 43 (34.4) | 50 (40) | 32 (25.6) | 125 (100) | |
| Total | 149 (46.4) | 123 (38.3) | 49 (15.3) | 321 (100) | |
| Relationship between age distribution of patients with osteoporosis and osteopenia based on femur t-score | 0.0 | ||||
| Less than 40 | 14 (58.3) | 9 (37.5) | 1 (4.2) | 24 (100) | |
| 40 - 60 | 90 (52.3) | 76 (44.2) | 6 (3.5) | 172 (100) | |
| More than 60 | 37 (29.8) | 73 (58.9) | 14 (11.3) | 124 (100) | |
| Total | 141 (44.1) | 158 (49.4) | 21 (6.6) | 320 (100) | |
| The relationship between age frequency distribution of patients with osteoporosis and osteopenia based on t-score of the forearm | - | ||||
| Less than 40 | 3 (42.9) | 3 (42.9) | 1 (14.3) | 7 (100) | |
| 40 - 60 | 20 (39.2) | 23 (45.1) | 8 (15.7) | 51 (100) | |
| More than 60 | 2 (6.5) | 9 (29) | 20 (64.5) | 31 (100) | |
| Total | 25 (28.1) | 35 (39.3) | 29 (32.6) | 89 (100) | |
| Relationship between age frequency distribution of patients with osteoporosis and osteopenia based on L1 density | 0.0 | ||||
| Less than 40 | 20 (83.3) | 4 (16.7) | 0 (0) | 24 (100) | |
| 40 - 60 | 120 (69.4) | 42 (24.3) | 11 (6.4) | 173 (100) | |
| More than 60 | 56 (44.8) | 43 (34.4) | 26 (20.8) | 125 (100) | |
Abbreviation: BMD, bone mineral density.
a Values are expressed as No. (%).
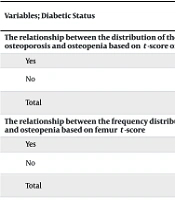
Diabetes was absent in most patients (97.5%, 314 individuals), while only 2.5% (8 individuals) had diabetes. Table 3 presents the prevalence of diabetes among patients with osteoporosis and osteopenia based on t-scores of the spine, femur, forearm, and L1 density.
| Variables; Diabetic Status | BMD Status | Total | Chi-square Test (P) | ||
|---|---|---|---|---|---|
| Normal | Osteopenia | Osteoporosis | |||
| The relationship between the distribution of the frequency of diabetes in patients with osteoporosis and osteopenia based on t-score of the spine | 0.327 | ||||
| Yes | 5 (71.4) | 2 (28.6) | 0 (0) | 7 (100) | |
| No | 144 (45.9) | 121 (38.5) | 49 (15.6) | 314 (100) | |
| Total | 149 (46.4) | 123 (38.3) | 49 (15.3) | 321 (100) | |
| The relationship between the frequency distribution of diabetes in patients with osteoporosis and osteopenia based on femur t-score | 0.667 | ||||
| Yes | 4 (57.1) | 3 (42.9) | 0 (0) | 7 (100) | |
| No | 137 (43.8) | 155 (49.5) | 21 (6.7) | 313 (100) | |
| Total | 141 (44.1) | 158 (49.4) | 21 (6.6) | 320 (100) | |
| The relationship between the distribution of the frequency of diabetes in patients with osteoporosis and osteopenia based on the t-score of the forearm | 0.040 | ||||
| Yes | 0 (0) | 0 (0) | 3 (100) | 3 (100) | |
| No | 25 (29.1) | 35 (40.7) | 26 (30.2) | 86 (100) | |
| Total | 25 (28.1) | 35 (39.3) | 29 (32.6) | 89 (100) | |
| The relationship between the frequency distribution of diabetes in patients with osteoporosis and osteopenia based on L1 density | 0.539 | ||||
| Yes | 5 (62.5) | 3 (37.5) | 0 (0) | 8 (100) | |
| No | 191 (60.8) | 86 (27.4) | 37 (11.8) | 314 (100) | |
| Total | 196 (60.9) | 89 (27.6) | 37 (11.5) | 322 (100) | |
Abbreviation: BMD, bone mineral density.
a Values are expressed as No. (%).
Chronic hypertension was present in a majority of patients (67.1%, 216 individuals), while 32.9% (106 individuals) did not have chronic hypertension. Table 4 shows the frequency of blood pressure findings among patients with osteoporosis and osteopenia based on t-scores of the spine, femur, forearm, and L1 density.
| Variables; Blood Pressure | BMD Status | Total | Chi-square Test | ||
|---|---|---|---|---|---|
| Normal | Osteopenia | Osteoporosis | |||
| Correlation of blood pressure in patients with osteoporosis and osteopenia based on the t-score of the spine | 0.025 | ||||
| Yes | 83 (38.6) | 91 (42.3) | 41 (19.1) | 215 (100) | |
| No | 66 (62.3) | 32 (30.2) | 8 (7.5) | 106 (100) | |
| Total | 149 (46.4) | 123 (38.3) | 49 (15.3) | 321 (100) | |
| Correlation of blood pressure frequency distribution in patients with osteoporosis and osteopenia based on femur t-score | 0.025 | ||||
| Yes | 83 (38.8) | 115 (53.7) | 16 (7.5) | 214 (100) | |
| No | 58 (54.7) | 43 (40.6) | 5 (4.7) | 106 (100) | |
| Total | 141 (44.1) | 158 (49.4) | 21 (6.6) | 320 (100) | |
| Correlation of blood pressure frequency distribution in patients with osteoporosis and osteopenia based on forearm t-score | 0.001 | ||||
| Yes | 11 (19.3) | 20 (35.1) | 26 (45.6) | 57 (100) | |
| No | 14 (43.8) | 15 (46.9) | 3 (9.4) | 32 (100) | |
| Total | 25 (28.1) | 35 (39.3) | 29 (32.6) | 89 (100) | |
| Correlation of blood pressure frequency distribution in patients with osteoporosis and osteopenia based on L1 density | 0.000 | ||||
| Yes | 115 (53.2) | 69 (31.9) | 32 (14.8) | 216 (100) | |
| No | 81 (76.4) | 20 (18.9) | 5 (4.7) | 106 (100) | |
| Total | 196 (60.9) | 89 (27.6) | 37 (11.5) | 322 (100) | |
Abbreviation: BMD, bone mineral density.
a Values are expressed as No. (%).
Regarding the measurement of bone density in the vertebrae, 15.3% of patients had osteoporosis, and 38.3% had osteopenia. In cases where bone density was measured in the femur region, osteoporosis was reported in 6.6% of cases and osteopenia in 49.3% of cases. For bone density measurement in the forearm region, only 89 patients were evaluated, with 32.6% having osteoporosis and 39.3% having osteopenia (Table 5).
| Bone Density | Spine | Femur | Forearm |
|---|---|---|---|
| Number | |||
| Less than -2.5 (osteoporosis) | 49 (15.3) | 21 (6.6) | 29 (32.6) |
| Between -2.5 and -1 (osteopenia) | 123 (38.3) | 158 (49.3) | 35 (39.3) |
| More than -1 (normal) | 149 (46.4) | 141 (44.1) | 25 (28.1) |
| Total | 321 (100) | 320 (100) | 89 (100) |
| Mean ± standard deviation | -1.095 ± 1.427 | 0.97 ± -1.10 | 1.29 ± -1.84 |
| Minimum-maximum | 3.1 - 5.3 | 1.6 - 4.2 | 0.9 - 5.4 |
Using a CT scan, bone density of the first lumbar vertebra revealed osteoporosis in 11.5% of patients and osteopenia in 27.6% of patients (Appendix 1, in Supplementary File). The majority of the samples (68.0%) represented stage 2 renal failure, followed by stage 1 (12 individuals, 3.7%), stage 3 (88 individuals, 27.3%), stage 4 (1 individual, 0.3%), and stage 5 (2 individuals, 0.6%) kidney failure. The relationship between the frequency of kidney failure stages and osteoporosis and osteopenia based on t-scores of the spine, femur, forearm, and L1 density is displayed in Appendix 2 in Supplementary File. Appendix 3 in Supplementary File demonstrates the relationship between kidney failure stages and t-scores of the spine, femur, forearm, and L1 density.
The average t-score for the spine was 1.095 ± 1.43, with the lowest t-score observed in patients with stage 4 and 5 renal insufficiency (data not shown). However, there was no statistically significant relationship between the stages of renal failure and spine bone density (P = 0.21), as indicated in Appendix 3 in Supplementary File. For the femur, the mean t-score was determined as 1.102 ± 0.97 (data not shown), with the lowest t-score observed in patients with stage 4 and 5 renal insufficiency. Similar to the spine, the relationship between the stages of renal failure and femur bone density was not statistically significant (P = 0.63), as presented in Appendix 3 in Supplementary File.
The average t-score for the forearm was 1.848 ± 1.29, and the highest t-score was found in patients with stage 1 kidney failure (data not shown). Despite patients with stage 4 and 5 kidney failure exhibiting osteoporosis or osteopenia, the relationship between the stages of kidney failure and femur bone density was not statistically significant (P = 0.29), according to Appendix 3 in Supplementary File.
The average L1 bone density measured through CT scan was 165.578 ± 55.304, with the lowest L1 bone density observed in patients with stage 4 and 5 kidney failure (data not shown). Patients with stage 4 and 5 renal failure demonstrated a statistically significant relationship between the stages of renal failure and L1 bone density (P = 0.027), as shown in Appendix 3 in Supplementary File.
L1 bone density measurement using the CT scan method for diagnosing osteoporosis demonstrated a sensitivity of 69.4%, specificity of 98.9%, positive predictive value of 91.9%, and negative predictive value of 94.7%. Pearson's test indicated a significant linear relationship (P ≤ 0.001) between L1 bone density measured by CT scan and spine bone density (R = 0.58). When the data were categorized into normal, osteopenia, and osteoporosis, Spearman's test revealed a significant linear relationship (P ≤ 0.001, Appendix 4, in Supplementary File) between L1 bone density by CT scan method and spine bone density (ρ = 0.751).
In comparison to femur bone density measurement, L1 bone density measurement in the diagnosis of osteoporosis exhibited a sensitivity of 47.6%, specificity of 90.9%, positive predictive value of 72.9%, and negative predictive value of 96.1%. Pearson's test demonstrated a significant linear relationship (P ≤ 0.001) between L1 bone density measured by CT scan and femur bone density (R = 0.44). After grouping the data into normal, osteopenia, and osteoporosis, Spearman's test indicated a significant linear relationship (P ≤ 0.001, Appendix 4, in Supplementary File) between L1 bone density and femur bone density (ρ = 0.48).
When comparing L1 bone density measurement by CT scan method to forearm bone density measurement in the diagnosis of osteoporosis, it showed a sensitivity of 8.13%, specificity of 0.95%, positive predictive value of 1.57%, and negative predictive value of 5.69%. Pearson's test revealed a significant linear relationship (P ≤ 0.001) between L1 bone density and forearm bone density (R = 0.41). Upon grouping the data into normal, osteopenia, and osteoporosis, a significant linear relationship (P = 0.05, Appendix 4, in Supplementary File) between L1 bone density by CT scan method and Spearman's correlation coefficient (ρ = 0.20) was observed.
In regard to diagnosing osteopenia, L1 bone density measurement by the CT scan method, compared to spine bone density measurement, demonstrated a sensitivity of 63.9%, specificity of 94.9%, positive predictive value of 88.6%, and negative predictive value of 81.0%. L1 bone density measurement by the CT scan method in the diagnosis of osteopenia showed a sensitivity of 41.1%, specificity of 85.2%, positive predictive value of 73.0%, and negative predictive value of 59.7%. Furthermore, L1 bone density measurement in the diagnosis of osteopenia, when compared to forearm bone density measurement, exhibited a sensitivity of 42.8%, specificity of 83.3%, positive predictive value of 62.5%, and negative predictive value of 69.2% (Appendix 5, in Supplementary File).
The results of logistic regression analysis revealed no significant relationship between age, MDRD (GFR), stage of kidney failure, gender, diabetes, and the occurrence of osteoporosis or osteopenia in patients with kidney failure. The only variable that demonstrated a significant relationship with the occurrence of osteoporosis or osteopenia in these patients was blood pressure. According to logistic regression analysis, high blood pressure increased the chances of having osteoporosis or osteopenia by 2.77 times. However, although a significant linear relationship was found (P ≤ 0.001, R = -0.187; Appendix 6, in Supplementary File) between age and bone density through Pearson's correlation, this relationship did not remain significant in the logistic regression analysis.
5. Discussion
When measuring bone density in the vertebrae, it was found that 15.3% of the patients were diagnosed with osteoporosis, and 38.3% had osteopenia. In the femur region, 6.6% of the cases were classified as osteoporosis, while osteopenia was reported in 49.3% of the cases. The forearm bone density measurement was conducted in only 89 patients, where 32.6% were diagnosed with osteoporosis, and 39.3% had osteopenia. Specifically, the measurement of bone density in the first lumbar vertebra using a CT scan revealed that 11.5% of the patients had osteoporosis, and 27.6% had osteopenia.
In a study conducted by Reddy et al., it was found that 42.8% of the patients had osteoporosis, while 40.2% had osteopenia (18). In our study sample, although the prevalence of osteopenia aligns with their findings, the prevalence of osteoporosis is lower. Additionally, their study indicated a higher prevalence of osteopenia and osteoporosis among individuals aged 60 and above. In our study, although there was a significant linear relationship (P ≤ 0.001) and R = -0.187 between age and bone density according to Pearson's correlation analysis, this relationship was not significant in the logistic regression analysis.
Furthermore, consistent with our findings, Hall et al. reported in a prospective cohort study that veterans with stage 3 CKD had an adjusted subdistribution hazard ratio (sdHR) of 1.07 (95% CI: 1.02 - 1.11) for fractures compared to those without CKD (10). Additionally, a recent meta-analysis reported that patients with CKD stage 5 had a significantly higher fracture risk, with a pooled hazard ratio (HR) of 2.63 (95% CI: 1.74 - 3.98), compared to individuals without CKD (19).
Based on the results of the present research, L1 bone density measurement using the CT scan method for diagnosing osteoporosis, compared to vertebral bone density measurement, demonstrated a sensitivity of 69.4%, specificity of 98.9%, positive predictive value of 91.9%, negative predictive value of 94.7%, and an overall test accuracy of 94.4% for the diagnosis of osteoporosis.
Similarly, L1 bone density measurement using the CT scan method for diagnosing osteopenia, compared to vertebral bone density measurement, showed a sensitivity of 63.9%, specificity of 94.9%, positive predictive value of 88.6%, negative predictive value of 81.0%, and an overall test accuracy of 82.9%. Comparing the measurement of L1 bone density to diagnosing osteoporosis and osteopenia with the measurement of vertebral bone density has yielded a high percentage of accurate diagnoses and acceptable test accuracy. Pearson's test revealed a significant linear relationship (P ≤ 0.001) between L1 bone density and vertebral bone density measurement, with a correlation coefficient (R) of 0.58. Additionally, after categorizing the data into three groups: Normal, osteopenia, and osteoporosis, Spearman's test showed a significant linear relationship (P ≤ 0.001) between L1 bone density and vertebral bone density measurement, with a correlation coefficient of 0.751.
These findings align with a study conducted by Kim et al. (2019), which classified patients into osteoporosis and non-osteoporosis groups and reported a sensitivity of 94.3% and a specificity of 87.5% (20). In contrast, when comparing the CT scan method for diagnosing osteoporosis through L1 bone density measurement to femur bone density measurement, the results yielded a sensitivity of 47.6%, specificity of 90.9%, positive predictive value of 72.9%, negative predictive value of 96.1%, and an overall test accuracy of 88.1%. Similarly, when diagnosing osteopenia, L1 bone density measurement using the CT scan method compared to femur bone density measurement exhibited a sensitivity of 41.1%, specificity of 85.2%, positive predictive value of 73.0%, negative predictive value of 59.7%, and an overall test accuracy of 63.4%.
Kim et al. also observed a significant correlation between BMD and L1 bone density measurement using the CT scan method (20), confirming the findings of the current study. Although Pearson's test demonstrated a significant linear relationship (P ≤ 0.001) between L1 bone density measured through the CT scan method and femur bone density measurement, the correlation coefficient (R) was lower compared to vertebral bone density measurement. This suggests that L1 bone density measured through the CT scan method might provide a more representative assessment of vertebral bone density.
Unlike the spine and femur, the diagnostic performance of L1 bone density obtained by CT scan in predicting osteoporosis in the forearm was poor, with a sensitivity of only 8.13% and a negative predictive value of 5.69%. These findings suggest that L1 bone density by CT scan is not a reliable tool for assessing osteoporosis in the forearm. For the diagnosis of osteopenia, L1 bone density measurement using the CT scan method compared to forearm bone density measurement demonstrated a sensitivity of 42.8%, specificity of 83.3%, positive predictive value of 62.5%, negative predictive value of 69.2%, and an overall test accuracy of 67.4%.
In a study conducted by Hendrickson et al., although the HU t-scores of lumbar vertebrae, including L1, were lower than DXA t-scores, they demonstrated high sensitivity (93%) and specificity (50%) in diagnosing osteoporosis (21). Although our study showed lower sensitivity compared to their findings, we achieved higher specificity. Hence, Hendrickson et al.'s study further supports the utility of this screening method.
Among patients with kidney failure, high blood pressure was the only variable significantly associated with the occurrence of osteoporosis or osteopenia. Logistic regression analysis revealed that individuals with high blood pressure had a 2.77-fold higher likelihood of developing osteoporosis or osteopenia. Jamshidian-Tehrani et al. also reported a significant relationship between blood pressure and osteoporosis in their study (22).
In this study, patients with stage 4 and 5 kidney insufficiency consistently exhibited osteoporosis or osteopenia across all diagnostic methods, including spine, femur, forearm, and L1 bone density measurements. Furthermore, higher stages of insufficiency were associated with a higher prevalence of osteopenia and osteoporosis. Although this relationship reached statistical significance only in L1 bone density measurement and not in spine, femur, and forearm measurements, the lack of significance in these measurements may be attributed to the small number of patients with stage 4 and 5 insufficiency.
5.1. Conclusions
In summary, this study reveals a significant association between L1 bone density and bone density measurements obtained from the spine, femur, and forearm in patients with kidney failure. Notably, the relationship is particularly prominent with spine measurements. Consequently, the non-invasive nature of L1 bone density measurement through CT scan, previously employed for purposes other than osteoporosis diagnosis, can serve as a screening tool for identifying osteoporosis and osteopenia. Implementing this approach not only leads to cost savings but also facilitates early detection of osteoporosis in these patients. This method demonstrated a high diagnostic accuracy, with an overall test validity of 94.4% in comparison to spine bone.
5.2. Limitations
While our study utilized DXA as the reference standard — consistent with current clinical guidelines for osteoporosis diagnosis in CKD (12) — the lack of comparison with QCT or histomorphometry may limit the mechanistic interpretation of HU values. The QCT, in particular, could provide 3D volumetric bone density data and differentiate trabecular vs. cortical bone loss (14). However, given the retrospective nature of our study and the routine clinical use of DXA, this limitation does not invalidate our primary finding that L1 HU measurements correlate significantly with DXA-based diagnoses. We encourage future studies to explore multi-modal imaging approaches in CKD patients. The retrospective design may affect data completeness (e.g., inconsistent follow-up CTs).
- Only 2.8% of our cohort (9/322) had stage 4 - 5 CKD, consistent with sampling challenges in other CKD studies (10).
- Single-center data may limit generalizability, though our hospital's national referral role mitigates this concern.
These factors should be considered when interpreting our results.
5.3. Future Studies
Future studies with larger sample sizes and more diverse populations are recommended to validate these findings. Moreover, evaluating the effects of medications, nutritional status, and other potential confounding factors on CT-derived bone density measurements would provide deeper insight into the utility of this screening approach.