1. Background
Vancomycin-associated acute kidney injury (AKI) is a common adverse event reported in pediatric patients treated with vancomycin (1). The incidence of this adverse effect ranges from 5% to 43%, depending on the patient population and the AKI criteria applied (2, 3). The AKI is a multifaceted clinical syndrome characterized by a rapid reduction (typically occurring over hours to weeks) in glomerular filtration, leading to the systemic accumulation of nitrogenous waste products such as creatinine and urea, and a concomitant disruption of fluid, electrolyte, and acid-base homeostasis (4). Long-term outcomes in AKI vary depending on severity, comorbidities, and recovery (4, 5). In children, this damage can significantly affect quality of life. Early recognition and prevention of AKI are crucial, as the associated mortality and morbidity are substantial (6). The use of appropriate medications to prevent AKI in children receiving vancomycin may help clinicians reduce renal damage and its complications, thereby improving patient outcomes (5, 7).
Montelukast is a highly specific antagonist of the leukotriene receptor, exhibiting strong binding affinity for the cysteinyl leukotriene 1 (CysLT1) receptor, which mediates the effects of leukotrienes D4 and E4 (8, 9). These potent lipid mediators are released from various cell populations, notably mast cells, and are pivotal in orchestrating the inflammatory cascade implicated in the pathophysiology of asthma and allergic rhinitis (10, 11). Montelukast has demonstrated significant antioxidant properties against kidney and liver damage. For example, studies have shown that montelukast can exert preventive and protective effects against AKI induced by doxorubicin and methotrexate, likely due to its capacity to counteract oxidative stress. Given these findings, it is hypothesized that montelukast may also have a protective effect against kidney damage caused by antibiotics such as vancomycin.
2. Objectives
The present study evaluated the protective effects of montelukast on vancomycin-associated AKI in children.
3. Methods
3.1. Study Setting and Population
This clinical trial was conducted at Amir Kabir Hospital in Arak, Iran, among children undergoing treatment with vancomycin. The sample size was determined a priori using power analysis: Based on an expected mean difference of 0.25 mg/dL in serum creatinine between groups, with a standard deviation of 0.25, α = 0.05, and power = 80%, a minimum of 18 participants per group was required. To account for potential dropouts, 20 participants were enrolled in each group (total n = 40). All enrolled patients (n = 40) completed the study without dropouts.
3.2. Randomization and Blinding
Randomization was performed using a computer-generated random sequence with a 1:1 allocation ratio. An independent researcher, not involved in patient care, data collection, or analysis, prepared sequentially numbered, opaque, sealed envelopes (SNOSE) to maintain allocation concealment. Each envelope contained the assigned group code, revealed only after participant enrollment. To minimize bias, triple blinding was implemented: Participants and their caregivers, treating physicians, and outcome assessors/statisticians were all blinded to group allocation. The montelukast tablets and standard treatment regimens were dispensed by a pharmacist not involved in patient assessment, preserving blinding integrity.
3.3. Inclusion and Exclusion Criteria
Inclusion criteria: (1) All children receiving vancomycin and (2) age older than 2 years. Exclusion criteria: (1) Lack of parental cooperation, (2) presentation with seizures, (3) history of kidney or liver disease, and (4) severe complications of montelukast and anaphylaxis.
3.4. Measurements
The study conditions were explained to all participants and their parents. Confidentiality was assured, and participants were informed that they could withdraw from the study at any time without consequence. Serum creatinine levels were assessed for all children in the study. The rate of change in creatinine was the criterion for AKI; an increase of ≥ 0.3 mg/dL from baseline was considered indicative of AKI. Patient information (age, gender, study group, underlying disease presence, AKI status, and serum creatinine) was recorded in a checklist.
Creatinine was measured at baseline (prior to vancomycin administration) and three days after the start of vancomycin. The primary outcome was the change in serum creatinine from baseline to day 3, used as a proxy for AKI. The AKI was defined according to the KDIGO criterion of an increase in serum creatinine ≥ 0.3 mg/dL within 48 hours. Other biomarkers of kidney injury (e.g., cystatin C, urine NGAL) were not assessed in this study and are recommended for future research.
Vancomycin was administered intravenously at 15 mg/kg per dose every 6 hours (total 60 mg/kg daily), with dosing adjustments based on serum trough levels and renal function. In the intervention group, children received vancomycin plus a 5 mg tablet of montelukast daily (administered to children aged ≥ 6 years, in accordance with pediatric dosing recommendations; lower doses were used for children under 6 years). In the control group, only vancomycin was administered, and efforts were made to keep all other medications as similar as possible between groups. At the end of the study, data were analyzed and compared between the two groups.
3.5. Ethical Considerations
The study received ethical approval from the Research Ethics Board of Arak University of Medical Sciences. All procedures adhered to the principles of the Helsinki Declaration. The ethical code for the study was IR.ARAKMU.REC.1400.316, and the registration number in the Iranian Registry of Clinical Trials (IRCTs) was IRCT20220519054919N1.
3.6. Statistical Analysis
Analyses were performed using Stata version 11 statistical software, with a 95% confidence level (CI). The chi-square test was used for comparing frequencies. For continuous variables, the Shapiro-Wilk test (or Kolmogorov-Smirnov, depending on sample size) assessed data normality. An independent samples t-test compared mean creatinine levels between groups, and a paired samples t-test compared creatinine levels within groups before and after intervention. When data distribution deviated from normality, the Mann-Whitney U test was used for between-group comparisons. The change score method was also used to evaluate the overall effect of the intervention.
4. Results
4.1. Age and Gender
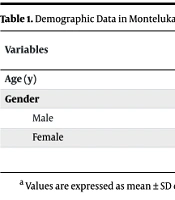
Among 40 cases, the mean ± SD age in the montelukast group was 5.95 ± 3.42 years, and in the control group was 5.42 ± 3.50 years. The male-to-female ratio in the montelukast group was 10/10 (50/50%), and in the control group was 8/12 (40/60%, Table 1).
| Variables | Groups | Total | |
|---|---|---|---|
| Montelukast | Control | ||
| Age (y) | 5.95 ± 3.42 | 5.42 ± 3.5 | 5.68 ± 3.46 |
| Gender | |||
| Male | 10 (50) | 8 (40) | 18 (45) |
| Female | 10 (50) | 12 (60) | 22 (55) |
a Values are expressed as mean ± SD or No. (%).
4.2. Mean and Standard Deviation of Creatinine Before and After Intervention
In the montelukast group, the mean ± SD serum creatinine before treatment was 0.68 ± 0.23 mg/dL and after treatment was 0.39 ± 0.13 mg/dL (P = 0.0001). In the control group, the mean ± SD serum creatinine before treatment was 0.55 ± 0.15 mg/dL and after treatment was 0.48 ± 0.15 mg/dL (P = 0.080, Table 2).
| Time | Groups | |
|---|---|---|
| Montelukast | Control | |
| Before | 0.68 ± 0.23 (0.58 - 0.78) | 0.55 ± 0.15 (0.48 - 0.62) |
| After | 0.39 ± 0.13 (0.33 - 0.45) | 0.48 ± 0.15 (0.41 - 0.55) |
| P-value | 0.0001 | 0.08 |
a Values are expressed as mean ± SD, 95% confidence level (CI).
5. Discussion
One of the significant challenges of vancomycin treatment in children is the risk of developing AKI. Various studies have explored different methods to mitigate renal complications, including the use of a low-risk medication such as montelukast. However, there has been no prior study on montelukast’s effect in reducing vancomycin-induced AKI. Consequently, the present study was conducted to address this gap.
Our findings indicate that montelukast, when administered alongside vancomycin, led to a reduction in serum creatinine and potentially prevented AKI. In the control group (vancomycin alone), creatinine increased in several cases after treatment, whereas the average creatinine level in the montelukast group was lower after intervention than before. This relationship has been explored in only a limited number of studies.
Teran et al. investigated the reduction of vancomycin-associated AKI with montelukast and suggested that montelukast administration during vancomycin therapy might offer a protective effect against AKI, potentially reducing patient morbidity and healthcare costs (10), which aligns with the findings of the present study.
Previous investigations into vancomycin use in AKI cases observed that continuing full-dose vancomycin in patients with AKI may enhance therapeutic target attainment while maintaining a comparable safety profile (12). Regarding montelukast in AKI, some studies have observed that montelukast may lower urea and serum creatinine levels, indicating improved kidney function (13). These studies also noted that pretreatment with montelukast often exerts a more significant influence than post-injury treatment (14), suggesting that early intervention is crucial for maximizing its protective effects (15). Additionally, prolonged montelukast administration has been associated with greater reductions in kidney damage (16).
In animal models, numerous preclinical studies have demonstrated the renoprotective properties of montelukast. For instance, Abdulredha and Majeed reported that montelukast administration in a murine sepsis model significantly reduced serum creatinine levels and attenuated histopathological kidney damage by modulating inflammatory pathways, particularly via the NF-κB signaling cascade (17). Similarly, Otunctemur et al. found that montelukast treatment mitigated renal tissue damage in rats subjected to unilateral ureteral obstruction, indicating its potential in obstructive nephropathy (13).
Clinical evidence supporting montelukast’s renoprotective effects is emerging. A scoping review by Sarmadian et al. concluded that montelukast is a safe and effective choice for improving renal function, particularly in early-stage kidney injury, by reducing inflammation and oxidative stress (8). Teran et al. found montelukast to be associated with reduced vancomycin-associated AKI, suggesting a potential role in preventing drug-induced renal injury (10).
The renoprotective effects of montelukast are primarily attributed to its anti-inflammatory and antioxidant properties. By antagonizing the CysLT1 receptor, montelukast inhibits the actions of leukotrienes implicated in the pathogenesis of various renal injuries. This inhibition leads to decreased neutrophil infiltration, reduced oxidative stress, and attenuation of pro-inflammatory cytokine release, collectively contributing to kidney protection (8). Tumor necrosis factor-alpha (TNF-α), released from activated macrophages, enhances the production of free oxygen radicals and the expression of adhesion factors in the vascular endothelium (18, 19). The TNF-α is also a key soluble factor released by mast cells, mediating urothelial responses (20). Supporting this, a cell culture study demonstrated that mast cells and TNF-α contribute to apoptosis in interstitial cystitis (IC) (21). Previous literature identified LTD4 receptors on human detrusor myocytes (22). LTD4, produced by mast cells within the detrusor muscle, induces a spasmogenic effect on the bladder, contributing to the symptoms and pain associated with IC. Montelukast, by blocking LTD4 receptors, exerts an anti-inflammatory effect via this pathway (23, 24). Based on this mechanistic understanding, it is plausible that montelukast could serve as an effective agent to mitigate vancomycin-associated AKI, particularly in pediatric patients.
5.1. Conclusions
The results of this study suggest that montelukast, when administered alongside vancomycin, may be associated with a reduction in serum creatinine in pediatric patients. While the montelukast group showed a decrease in mean creatinine levels after the intervention, the control group exhibited only a slight, non-significant increase in some cases. Given the small sample size, short follow-up period, and single-center design, these findings should be interpreted with caution. Future studies with larger cohorts, longer follow-up periods, and evaluation of different montelukast dosing regimens are needed to confirm its potential role in preventing AKI.
While our study observed a reduction in serum creatinine in the montelukast group, it is important to note that the control group did not demonstrate statistically significant progression of AKI. Therefore, the clinical significance of the observed creatinine reduction should be interpreted cautiously. These findings suggest a potential protective effect of montelukast on kidney function, but further studies with larger sample sizes are needed to confirm its clinical relevance.
5.2. Limitations
This study had several limitations. First, non-cooperation from parents posed a challenge, although this was partially mitigated by emphasizing the benefits of participation. Second, the relatively small sample size limited the generalizability of the findings; this was addressed to some extent by extending the sampling period. Additional limitations include the lack of long-term follow-up, reliance on creatinine alone as a biomarker, the presence of potential confounding variables that were not fully controlled, and the absence of pharmacokinetic data such as vancomycin trough levels. Furthermore, the single time-point assessment of serum creatinine (day 3) may not capture delayed-onset AKI. Future studies should consider longer follow-up periods (e.g., 7 - 14 days) and include additional renal biomarkers such as cystatin C and NGAL for a more comprehensive assessment of kidney function. Moreover, the absence of adverse event reporting in our study warrants attention; future research should systematically monitor and report any potential side effects associated with montelukast administration.