1. Background
Ischemic heart disease (IHD) remains one of the leading causes of morbidity and mortality worldwide, imposing a substantial burden on global healthcare systems (1). As a clinical manifestation of atherosclerotic cardiovascular disease (CVD), IHD is characterized by progressive narrowing and obstruction of the coronary arteries, ultimately resulting in myocardial ischemia, angina, and frequently myocardial infarction (2). Coronary artery bypass grafting (CABG) is among the most established surgical interventions for patients with advanced or extensive coronary artery disease (CAD). It has been shown to effectively restore myocardial perfusion and improve survival, quality of life, and functional capacity in appropriately selected patients (3). Given the systemic nature of atherosclerosis, patients with significant coronary artery involvement often exhibit concurrent atherosclerotic changes in other vascular beds, particularly the carotid arteries. This coexistent pathology holds critical clinical importance in the setting of cardiac surgery, where impaired cerebral perfusion can lead to serious neurologic complications (4). Carotid artery disease, especially stenosis, has been recognized as a significant predictor of perioperative stroke, transient ischemic attack (TIA), and other neurologic sequelae in individuals undergoing cardiac surgery, including CABG (5). Disease of the external carotid artery (ECA) may serve as an indirect marker of overall atherosclerotic burden and is particularly relevant as a potential source of embolic stroke during or after surgical manipulation. Both symptomatic and asymptomatic carotid stenosis can compromise cerebral blood flow during cardiopulmonary bypass and serve as a source of atheroemboli, thereby increasing the risk of ischemic brain injury in the perioperative period (6). Early detection of carotid artery stenosis (CAS) is thus vital in the preoperative evaluation of CABG candidates, especially among those with known risk factors such as advanced age, hypertension (HTN), diabetes mellitus (DM), dyslipidemia, and smoking (7). Although the relationship between carotid and CAD is well-documented, routine preoperative screening for carotid stenosis in CABG candidates is not universally implemented. Nonetheless, targeted screening based on clinical history, physical examination, and selective use of non-invasive imaging can aid in identifying patients at increased risk for neurologic complications during surgery (8). In this regard, Doppler ultrasonography of the carotid arteries serves as a valuable, non-invasive diagnostic modality. It is widely accessible, cost-effective, and repeatable, and provides detailed information on the presence, location, and severity of carotid stenosis — information that can critically inform surgical planning and risk stratification (9). Given the notable prevalence of asymptomatic CAS among patients undergoing CABG, integrating routine carotid Doppler ultrasonography into preoperative assessments may help reduce the incidence of cerebrovascular accidents (CVAs) and improve neurological outcomes. Early identification of high-risk individuals allows for the timely implementation of preventive strategies such as carotid interventions, modifications to surgical planning, or intensified postoperative surveillance.
2. Objectives
Accordingly, the present study was designed to assess the prevalence of CAS in patients scheduled for CABG using Doppler ultrasound and to identify associated cardiovascular risk factors predictive of its presence and severity. The findings aim to underscore the clinical utility of routine carotid screening in CABG candidates and to support the development of evidence-based protocols for minimizing postoperative neurologic complications.
3. Methods
3.1. Study Design and Setting
This descriptive cross-sectional study was conducted using a census sampling method on patients referred for CABG at selected hospitals in Zahedan, Iran. The study was carried out over a one-year period, from April 2019 to April 2020. All eligible patients scheduled for CABG during this time frame were included in the study.
3.2. Study Population and Criteria
A total of 300 patients who were candidates for CABG were enrolled in the study. The sample consisted of 151 males (50.3%) and 149 females (49.7%). All participants were referred to the hospitals with clinical symptoms suggestive of CAD and had undergone coronary angiography, which confirmed the presence of significant coronary artery lesions not amenable to medical management or percutaneous interventions. Only those patients who required surgical revascularization (CABG) based on the angiographic findings were included in the study. Patients who had previously undergone carotid artery surgery, those with known carotid artery disease, or individuals who declined participation after the consent process were excluded from the study.
3.3. Data Collection Procedures
Data collection was performed using a combination of field methods and library/document review. The primary data sources included: Patient medical records (for demographic and clinical data), patient interviews (to confirm history and clarify risk factors), and structured questionnaires (to systematically document relevant variables).
3.4. Carotid Artery Ultrasound Assessment
All enrolled patients underwent preoperative carotid artery Doppler ultrasonography to evaluate for carotid stenosis. This examination was conducted by an experienced radiologist involved in the research team. A GE ultrasound device equipped with a linear array probe operating at a frequency of 5.7 MHz was used for imaging. The examination was performed in two main phases using duplex ultrasonography. First, gray-scale B-mode imaging was employed to visualize the anatomical course and structural characteristics of the common carotid artery (CCA), internal carotid artery (ICA), and ECA, allowing for documentation of the full length of the cervical carotid arteries. Subsequently, color Doppler and spectral Doppler techniques were used to assess blood flow disturbances and evaluate the presence and severity of carotid stenosis. Based on peak systolic velocity and waveform analysis, stenosis was classified into four categories: No stenosis, mild (< 50% luminal narrowing), moderate (50 - 70% narrowing), and severe (> 70% narrowing). Findings from both imaging phases were systematically recorded using a pre-designed checklist for further analysis.
3.5. Risk Factor Assessment
Demographic information and potential risk factors associated with CAS were collected, including age, sex, history of chronic diseases such as DM, HTN, and hyperlipidemia (HLP), as well as behavioral risk factors like cigarette smoking and substance abuse. Additional data on relevant medical history, including cardiovascular comorbidities, were also gathered. This information was obtained through patient interviews and review of medical records, then systematically recorded in a standardized checklist.
3.6. Ethical Considerations
Written informed consent was obtained from all participants, and data confidentiality was strictly maintained. The study was approved by the Ethics Committee of Zahedan University of Medical Sciences (IR.ZAUMS.REC.1399.268).
3.7. Data Analysis
Data were analyzed using SPSS v26. Descriptive statistics summarized participant characteristics, chi-square tests assessed associations between categorical variables, and binary logistic regression evaluated the link between risk factors and carotid stenosis. A P-value < 0.05 was considered significant.
4. Results
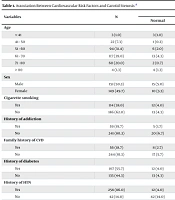
In this cross-sectional study, a total of 300 candidates for CABG underwent carotid artery Doppler ultrasonography to assess the presence and severity of CAS. Furthermore, the associations and correlations between various cardiovascular risk factors and CAS were evaluated (Tables 1 and 2).
| Variables | N | Carotid Stenosis | P-Value | ||
|---|---|---|---|---|---|
| Normal | Mild | Moderate | |||
| Age | < 0.001 | ||||
| < 41 | 3 (1.0) | 3 (1.0) | 0 (0.0) | 0 (0.0) | |
| 41 - 50 | 22 (7.3) | 1 (0.3) | 9 (3.0) | 12 (4.0) | |
| 51 - 60 | 94 (31.4) | 6 (2.0) | 66 (22.0) | 22 (7.4) | |
| 61 - 70 | 117 (39.0) | 13 (4.3) | 58 (19.4) | 46 (15.3) | |
| 71 - 80 | 60 (20.0) | 2 (0.7) | 40 (13.3) | 18 (6.0) | |
| > 80 | 4 (1.3) | 4 (1.3) | 0 (0.0) | 0 (0.0) | 0.098 |
| Sex | |||||
| Male | 151 (50.3) | 15 (5.0) | 80 (26.7) | 56 (18.6) | |
| Female | 149 (49.7) | 10 (3.3) | 97 (32.4) | 42 (14.0) | |
| Cigarette smoking | 0.044 | ||||
| Yes | 114 (38.0) | 12 (4.0) | 57 (19.0) | 45 (15.0) | |
| No | 186 (62.0) | 13 (4.3) | 120 (40.0) | 53 (17.7) | |
| History of addiction | 0.013 | ||||
| Yes | 59 (19.7) | 5 (1.7) | 44 (14.7) | 10 (3.3) | |
| No | 241 (80.3) | 20 (6.7) | 133 (44.3) | 88 (29.3) | |
| Family history of CVD | 0.013 | ||||
| Yes | 56 (18.7) | 8 (2.7) | 38 (12.7) | 10 (3.3) | |
| No | 244 (81.3) | 17 (5.7) | 139 (46.3) | 88 (29.3) | |
| History of diabetes | < 0.001 | ||||
| Yes | 167 (55.7) | 12 (4.0) | 74 (24.7) | 81 (27.0) | |
| No | 135 (44.3) | 13 (4.3) | 103 (34.3) | 17 (5.7) | |
| History of HTN | < 0.001 | ||||
| Yes | 258 (86.0) | 12 (4.0) | 148 (49.3) | 98 (32.7) | |
| No | 42 (14.0) | 42 (14.0) | 13 (4.3) | 29 (9.7) | |
| History of HLP | 0.001 | ||||
| Yes | 174 (58.0) | 13 (4.3) | 118 (39.3) | 43 (14.3) | |
| No | 126 (42.0) | 12 (4.0) | 59 (19.7) | 55 (18.3) | |
| History of CVA | < 0.001 | ||||
| Yes | 17 (5.7) | 3 (1.0) | 0 (0.0) | 14 (4.7) | |
| No | 283 (94.3) | 22 (7.3) | 177 (59.0) | 84 (28.0) | |
| History of TIA | 0.002 | ||||
| Yes | 11 (3.7) | 4 (1.3) | 7 (2.3) | 0 (0.0) | |
| No | 289 (96.3) | 21 (7.0) | 170 (56.7) | 98 (32.7) | |
| History of MI | 0.019 | ||||
| Yes | 153 (51.0) | 13 (4.3) | 79 (26.3) | 61 (20.3) | |
| No | 147 (49.0) | 12 (4.0) | 98 (32.7) | 37 (12.3) | |
Abbreviations: CVD, cardiovascular disease; HTN, hypertension; HLP, hyperlipidemia; CVA, cerebrovascular accident; TIA, transient ischemic attack.
a Values are expressed as No. (%).
| Variables | Β | P-Value | Exp (Β) | CI |
|---|---|---|---|---|
| Cigarette smoking | 0.440 | 0.28 | 1.56 | 0.68 - 3.51 |
| Diabetes | 0.336 | 0.42 | 1.4 | 0.616 - 3.17 |
| Family history of CVD | -0.800 | 0.08 | 0.44 | 0.18 - 1.1 |
| History of CVA | -0.933 | 0.66 | 0.39 | 0.10 - 1.47 |
| History of MI | 0.040 | 0.91 | 1.02 | 0.42 - 2.17 |
| HLP | 0.260 | 0.52 | 1.3 | 0.57 - 2.96 |
Abbreviations: CVD, cardiovascular disease; CVA, cerebrovascular accident; HLP, hyperlipidemia.
The analysis revealed that the prevalence of CAS among the study population was [insert %/number]. Patients with significant stenosis demonstrated a higher frequency of traditional cardiovascular risk factors, including HTN, DM, HLP, and smoking history, compared with those without stenosis (P < 0.05 for all). A statistically significant positive correlation was observed between the degree of carotid stenosis and the presence of these risk factors. Additionally, age and male sex were significantly associated with an increased risk of CAS.
Overall, the findings indicate that CAS is common in CABG candidates and is strongly correlated with established cardiovascular risk factors.
5. Discussion
This study examined the prevalence of CAS among candidates for CABG in hospitals in Zahedan between 2019 and 2020 and assessed its relationship with conventional cardiovascular risk factors. Among the 300 patients evaluated, the largest age subgroup was 61 - 70 years, and the sex distribution was nearly balanced (151 males and 149 females). The univariate analysis revealed significant associations between CAS and several risk factors, including age, family history of CVD, HTN, HLP, cigarette smoking, substance use, and histories of TIA and CVA. These findings are consistent with the widely accepted view that CAS, as a manifestation of systemic atherosclerosis, shares common pathophysiological pathways with other forms of vascular disease. However, multivariate logistic regression did not identify any of these risk factors as independently associated with CAS. This suggests that while these variables may demonstrate significant associations individually, they lack sufficient predictive power when modeled together. Such results underscore the multifactorial and interdependent nature of atherosclerotic disease, where overlapping risk profiles may obscure the individual contribution of specific factors.
Similar patterns and contrasts with the present findings have been reported in previous studies. Montazerghaem (10) observed no significant association between CAS greater than 50% and major cardiovascular risk factors in patients over 60 years, which differs from the current study where a clear relationship between these risk factors and CAS was identified. Conversely, Park et al. (11) reported that CAS prevalence varied according to different combinations of risk variables, reinforcing the importance of individualized risk profiling—a finding that aligns with the observed correlations in this study.
Age has been consistently highlighted as a significant determinant of CAS. While the current investigation confirmed that advancing age, particularly in individuals aged ≥ 50 - 65 years, is associated with increased stenosis prevalence, this observation is in agreement with studies by Ranjan et al. (12), Sadeghi et al. (13), Cheng et al. (14), and Alsalmi and Abdeen (15), all of which reported odds ratios (ORs) up to 1.79. These findings collectively underscore the cumulative impact of systemic atherosclerosis with aging.
Regarding HTN, the present results parallel those of Ranjan et al. (12), Sadeghi et al. (13), and Alsalmi and Abdeen (15), who demonstrated that uncontrolled HTN is a strong predictor of CAS, with reported ORs around 2.38. Likewise, DM was confirmed as a significant risk factor in both the current study and previous reports, where poorly controlled DM was associated with an OR of 2.51, reflecting the vascular damage caused by chronic hyperglycemia.
Further comparisons reveal that chronic kidney disease (CKD), as identified by Sadeghi et al. (13), independently predicts severe carotid disease due to its link with endothelial dysfunction and vascular calcification — an observation that complements the present findings. Additionally, the strong association between multivessel CAD and CAS reported by Ranjan et al. (12) (OR = 3.79) is consistent with this study’s demonstration of the systemic nature of atherosclerosis. Finally, dyslipidemia, documented by both Ranjan et al. (12) and Sadeghi et al. (13) as increasing the risk of stenosis (OR = 2.19), also aligns with the current results, further supporting the role of lipid abnormalities in atherosclerotic plaque development.
Overall, while certain studies, such as that by Montazerghaem (10), report divergent findings, most prior investigations corroborate the associations identified in this study, particularly with respect to age, HTN, diabetes, multivessel CAD, and dyslipidemia as significant contributors to CAS.
Smoking remains a well-known and powerful contributor to vascular disease, and its impact on CAS is no exception. Cheng et al. (14) reported smoking as an independent risk factor for CAS in Chinese patients undergoing CABG, linking it to the damage smoking causes to blood vessels through inflammation and injury to the vessel lining. These findings echo what was observed in this study, where smoking history was closely tied to more severe stenosis, highlighting the importance of smoking cessation, particularly in patients preparing for heart surgery.
A history of stroke also stood out as an important warning sign. Sadeghi et al. (13) found that patients who had suffered a CVA were more likely to have significant CAS. This makes intuitive sense, as both conditions often share the same underlying cause — widespread atherosclerosis. The current findings support this connection, reinforcing why patients with a previous stroke should undergo careful carotid evaluation before undergoing CABG. Detecting stenosis in these patients is crucial because it can shape both surgical planning and perioperative care.
The risk becomes even more pronounced when multiple health problems are present together. According to Ranjan et al. (12), having two or more conditions — such as diabetes, HTN, kidney disease, or high cholesterol — can quadruple the risk of developing CAS. This study found a similar pattern: Patients with a combination of risk factors were far more likely to have moderate to severe narrowing. This underscores the need to look at patients as a whole, not just by individual risk factors.
These findings strengthen the case for routine preoperative screening with carotid duplex ultrasonography in patients scheduled for CABG, especially those over 60 or those with multiple uncontrolled risk factors. Carotid Doppler ultrasound is a simple, safe, and cost-effective way to detect narrowing early, allowing doctors to take preventive steps when needed.
When severe narrowing (≥ 70%) is found, particularly if it affects both sides or is causing symptoms, doctors may consider combined surgical treatment — addressing both the heart and carotid arteries. However, the decision is not straightforward. The CABACS trial by Knipp et al. (16) showed no clear survival or stroke benefit from doing both surgeries together at five years, suggesting that the choice must be individualized. Patient age, overall health, and the specific characteristics of the stenosis all play a role in determining the best approach.
Adding to the weight of these findings, da Rosa et al. (17) showed that the presence of CAS was linked to a higher risk of death after CABG, making it clear that CAS is not just a local problem but a sign of widespread vascular disease.
Overall, these insights reinforce that detecting CAS early and understanding each patient’s risk profile is essential. A tailored strategy, whether it involves better medical control, lifestyle changes, or selective surgery, can make a real difference in reducing complications and improving long-term outcomes in patients facing CABG.
5.1. Conclusions
In conclusion, while CAS was found to be associated with several cardiovascular risk factors in univariate analyses, none of these factors independently predicted stenosis in multivariate regression. Age remains a prominent factor associated with increased severity of stenosis, whereas sex does not appear to be a significant determinant. These findings suggest the need for routine carotid screening in older patients undergoing CABG, regardless of the presence or absence of individual cardiovascular risk factors. Further multicenter, prospective studies are recommended to explore causal pathways and refine screening criteria to improve clinical outcomes in this high-risk population.
5.2. Limitations
Several limitations should be acknowledged. First, this was a single-center study, which may limit the generalizability of our findings to other populations. Second, the cross-sectional design precludes the establishment of temporal or causal relationships between risk factors and carotid stenosis. Third, although a range of risk factors was considered, other potential confounders such as physical activity, diet, and genetic predisposition were not assessed. Lastly, ultrasound-based assessment of stenosis, while non-invasive and widely used, may be subject to inter-observer variability.