1. Background
Neonatal jaundice, characterized by elevated serum bilirubin levels, affects approximately 60% of term and 80% of preterm infants within the first week of life (1). This condition is more prevalent and severe in low birth weight (LBW) infants (< 2500 g) due to immature hepatic and gastrointestinal systems, which increase the risk of complications such as kernicterus and irreversible neurological damage (2). Phototherapy is the standard treatment for neonatal hyperbilirubinemia, effectively reducing bilirubin by converting it to water-soluble isomers (3). However, phototherapy may cause side effects including hyperthermia, dehydration, and disruption of mother-infant bonding, potentially interfering with breastfeeding (4).
Probiotics, live microorganisms that promote gut microbiota balance, have been proposed as an adjunctive therapy by enhancing gut motility and reducing enterohepatic circulation of bilirubin, thereby accelerating its clearance (5, 6).
2. Objectives
Despite mixed results in previous studies regarding their efficacy (7, 8), this study aims to investigate the effectiveness of Pedilact (containing Lactobacillus reuteri, L. rhamnosus, and Bifidobacterium infantis) combined with phototherapy in reducing bilirubin levels and the duration of phototherapy in LBW infants with jaundice at Imam Reza Hospital, Mashhad.
3. Methods
3.1. Study Design
This double-blind randomized clinical trial was conducted in the Neonatal Intensive Care Unit (NICU) of Imam Reza Hospital, Mashhad, from 2023 to 2024. The study was approved by the Ethics Committee of Mashhad University of Medical Sciences (IR.MUMS.MEDICAL.REC.1403.342) and registered in the Iranian Registry of Clinical Trials (IRCT20250103064264N1).
- Inclusion criteria: The LBW (1000 - 2500 g) infants younger than 30 days admitted to the NICU with jaundice, without infectious, cardiac, gastrointestinal, renal, or respiratory diseases requiring ventilation, without congenital anomalies or jaundice due to hemolysis, and with parental consent.
- Exclusion criteria: Development of sepsis or withdrawal of parental consent.
3.2. Interventions
- Intervention group (n = 36): Received 5 drops of Pedilact (manufactured by Zist Takhmir Pharmaceutical Company, Iran) daily for 3 days alongside standard phototherapy.
- Control group (n = 36): Received 5 drops of distilled water daily for 3 days alongside standard phototherapy.
3.3. Bilirubin Measurement
Bilirubin levels were measured at 12 to 24, 48, and 72 hours post-intervention using the photometric method with the Prestige 24i autoanalyzer (Japan). Venous blood samples were centrifuged to obtain serum. Commercial kits from Pars Azmun Company (Iran), based on specific biochemical reactions and absorbance, were used for bilirubin assay. All measurements followed manufacturer instructions and were validated by internal quality control.
3.4. Statistical Analysis
Sample size was calculated based on Jenabi et al. (9), assuming 80% power and 0.05 significance level, resulting in 36 infants per group. Data analysis was performed using SPSS version 27 (IBM Corp., Armonk, NY, USA). Continuous variables were analyzed using independent t-tests, and categorical variables using Chi-square tests. A p-value less than 0.05 was considered statistically significant.
3.5. Ethical Considerations
Informed consent was obtained from parents, patient data were kept confidential, and no additional costs were imposed on participants.
4. Results
4.1. Baseline Characteristics
At the start of the study, the two groups — probiotic and control — were comparable across a range of demographic and clinical variables, indicating successful randomization and minimizing potential confounding factors. There were no statistically significant differences in the mean age of the infants (P = 1) or their sex distribution (P = 1), suggesting that both groups had a similar gender ratio and age range. Birth weight, an important predictor of neonatal outcomes, showed no significant difference between groups (mean ± SD: 1980.83 ± 585.57 g in the probiotic group vs. 2104.72 ± 595.51 g in the control group; P = 0.87), indicating that the infants’ size at birth was balanced.
Additionally, blood group distributions were similar (P = 0.62), reducing the likelihood that blood type-related factors influenced outcomes. Use of antibiotics prior to or during the study did not differ significantly between groups (P = 1), which is important as antibiotic exposure can impact gut microbiota and potentially bilirubin metabolism. Delivery type (vaginal vs. cesarean) was also comparable (P = 1), controlling for differences in neonatal health linked to mode of birth. Finally, gestational age, a critical factor in neonatal development and jaundice risk, showed no significant difference (P = 0.83), confirming that the prematurity level was evenly distributed.
Overall, these baseline similarities suggest that any observed differences in outcomes are likely attributable to the intervention rather than pre-existing group differences.
4.2. Bilirubin Levels
4.2.1. 12 - 24 Hours
In the probiotic group, 44.4% (16 infants) had bilirubin levels below 10 mg/dL, compared to 25% (9 infants) in the control group. The mean bilirubin level was 9.13 ± 3.49 mg/dL in the probiotic group versus 11.9 ± 3.13 mg/dL in the control group (mean ± SD). This difference was not statistically significant (P = 0.0001).
4.2.2. 48 Hours
In the probiotic group, 69.4% (25 infants) had bilirubin levels below 10 mg/dL, compared to 22.2% (8 infants) in the control group. The mean bilirubin level was 8.06 ± 2.76 mg/dL in the probiotic group versus 12.19 ± 3.08 mg/dL in the control group (mean ± SD). This difference was statistically significant (P = 0.0001).
4.2.3. 72 Hours
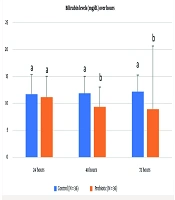
In the probiotic group, 75% (27 infants) had bilirubin levels below 10 mg/dL, compared to 16.7% (6 infants) in the control group. The mean bilirubin level was 6.86 ± 2.02 mg/dL in the probiotic group versus 11.58 ± 3.26 mg/dL in the control group (mean ± SD). This difference was also statistically significant (P = 0.0001) (Table 1).
| Time Point (hr) | Control | Probiotic | P-Value | ||
|---|---|---|---|---|---|
| Mean ± SD | % < 10 mg/dL | Mean ± SD | % < 10 mg/dL | ||
| 12 to 24 hours | 11.9 ± 3.13 | 25% (9 infants) | 9.13 ± 3.49 | 44.4% (16 infants) | 0.0001 |
| 48 | 12.19 ± 3.08 | 22.2% (8 infants) | 8.06 ± 2.76 | 69.4% (25 infants) | 0.0001 |
| 72 | 11.58 ± 3.26 | 16.7% (6 infants) | 6.86 ± 2.02 | 75% (27 infants) | 0.0001 |
Table 1 presents the mean serum bilirubin levels and the percentage of infants with levels below 10 mg/dL at 12 to 24, 48, and 72 hours post-intervention in both groups. The analysis revealed a statistically significant difference between the groups at all measured time points (12 to 24, 48, and 72 hours), with a P-value of < 0.0001 for each comparison. The probiotic group exhibited significantly lower bilirubin levels and a higher proportion of infants with bilirubin < 10 mg/dL. A P-value of less than 0.05 was considered statistically significant.
4.2.4. Other Findings
No significant differences were found between groups in hemoglobin (P = 0.30) or hematocrit levels (P = 0.96), suggesting that probiotic administration did not adversely affect these hematologic parameters and supports its safety in this population.
5. Discussion
This study demonstrates that Pedilact, as an adjunct to phototherapy, significantly reduces bilirubin levels in LBW infants with jaundice, particularly at 48 and 72 hours post-treatment. These findings align with previous research, such as Ramadan et al., who reported a significant reduction in bilirubin levels in term infants receiving probiotics alongside phototherapy (7). Similarly, Mutlu found that L. rhamnosus GG reduced bilirubin levels in term infants by 36 hours (8). The present study extends these findings to LBW infants, a population at higher risk for severe hyperbilirubinemia due to immature physiological systems (2).
The mechanism by which probiotics reduce bilirubin levels likely involves modulation of gut microbiota, enhancement of gut motility, and reduction of enterohepatic circulation. Probiotics such as Lactobacillus and Bifidobacterium species may decrease β-glucuronidase activity, which deconjugates bilirubin in the gut, thereby reducing its reabsorption (5, 10). Additionally, probiotics may accelerate meconium passage, facilitating bilirubin excretion (6). These mechanisms are particularly relevant in LBW infants, who often have delayed gut colonization and impaired bilirubin metabolism (11).
Comparatively, a study by Saeidi conducted at Shahid Beheshti University of Medical Sciences investigated the role of probiotics in preterm infants with jaundice and found a significant reduction in bilirubin levels and phototherapy duration with probiotic supplementation (12).
Ahmad Shah Farhat showed that a five-day probiotic treatment significantly reduced bilirubin levels in preterm neonates without prior phototherapy. In those with previous phototherapy, a significant reduction was seen only on day six. Probiotics had no significant effect on weight gain, supporting their safety and potential as an adjunct therapy before phototherapy in neonatal jaundice management (13). This corroborates our findings, highlighting the potential of probiotics to enhance phototherapy outcomes in vulnerable populations.
Another study by Armanian et al. in preterm infants (< 1500 g) reported improved feeding tolerance and reduced bilirubin levels with prebiotic supplementation, further supporting the role of gut microbiota modulation in jaundice management (11). However, conflicting results exist. Zahed Pasha et al. (2017) found no significant difference in phototherapy duration with probiotic use in term infants, suggesting that efficacy may vary based on probiotic strains, dosages, or patient characteristics (14). Differences in study populations (e.g., term vs. preterm infants) and probiotic formulations may explain these discrepancies. For instance, our study used Pedilact, which contains multiple probiotic strains, potentially offering synergistic effects compared to single-strain probiotics used in other studies (15).
The clinical implications of our findings are significant. Reducing bilirubin levels more rapidly can shorten hospital stays, decrease healthcare costs, and mitigate the emotional and financial burden on families. Moreover, probiotics like Pedilact are cost-effective and widely available, making them a practical adjunctive therapy. However, the lack of significant differences at 24 hours suggests that probiotics may require a longer duration to exert measurable effects, possibly due to the time needed for gut colonization (16).
Limitations of this study include the short follow-up period (72 hours), which precludes assessment of long-term outcomes or potential adverse effects of probiotics. Additionally, the study focused on LBW infants, and results may not be generalizable to term infants or those with hemolytic jaundice. Strengths include the double-blind design, adequate sample size, and standardized probiotic formulation, which enhance the reliability of the findings.
Future research should explore the long-term safety and efficacy of probiotics in neonatal jaundice, including potential effects on neurodevelopmental outcomes. Comparative studies evaluating different probiotic strains and dosages could further optimize treatment protocols. Additionally, cost-effectiveness analyses, as suggested by Saeidi (17), could provide insights into the economic benefits of probiotic supplementation in resource-constrained settings.
5.1. Conclusions
The use of Pedilact as an adjunct to phototherapy significantly accelerates bilirubin reduction in LBW infants with jaundice, potentially reducing the duration of hospitalization. This cost-effective and accessible intervention offers a promising approach to managing neonatal jaundice in high-risk populations.
5.2. Recommendations
- Conducting studies with longer follow-up to assess the safety and long-term effects of probiotics.
- Investigating the efficacy of different probiotic strains and dosages in diverse neonatal populations.
- Performing cost-effectiveness analyses to evaluate the economic impact of probiotic supplementation in jaundice management.