1. Context
Apolipoprotein E (APOE) is a glycoprotein that plays a critical role in lipid transport and metabolism. It is encoded by the APOE gene, which primarily exists in three allelic variants: ε2, ε3, and ε4 (1). Among these, the ε4 allele is widely recognized as a key genetic risk factor for Alzheimer’s disease, where it promotes neuroinflammation and disrupts neuronal repair processes (2). Apolipoprotein E has a well-known function in lipid metabolism and neurodegenerative diseases. However, recent studies have revealed its influence in oncology, particularly in breast cancer, as it induces tumor progression while also contributing to cancer-related cognitive impairment (CRCI) (3). Apolipoprotein E three isoforms, ε2, ε3, and ε4, have distinct effects on tumor biology and cognitive outcomes (4). Apolipoprotein E influences the tumor microenvironment (TME) by modulating immune responses, particularly through macrophage polarization (5, 6). It can either act as a tumor suppressor or an activator factor (7-9). Also, the ε4 allele has been found in association with cognitive impairment in chemotherapy survivors, indicating APOE’s role in cancer treatment-related neurotoxicity (10). These findings highlight the potential of APOE genotyping to guide clinical pharmacy practice and enable clinicians to design personalized treatment plans, optimize medication regimens, and mitigate cognitive side effects. This brief review synthesizes current knowledge on APOE’s role in breast cancer and CRCI, emphasizing its applications in pharmacy practice to enhance patient-centered care and rational drug use.
2. Apolipoprotein E in Breast Cancer
Apolipoprotein E plays a key role in creating cancer’s TME, mainly by modulating immune responses and controlling macrophage polarization (11). Apolipoprotein E is highly expressed in solid tumors, including breast cancer, and significantly regulates macrophage polarization, a key component of the TME (12, 13). In this pathway, macrophages are polarized toward an M2-like phenotype to induce an immunosuppressive microenvironment, facilitating tumor growth and metastasis (14). An elevated level of M2-like macrophages within the TME is correlated with poorer prognosis in breast cancer, highlighting the influential role of APOE in tumor progression (15).
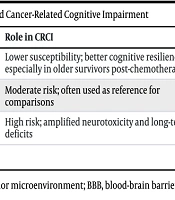
The function of APOE in the lipid metabolism pathway significantly impacts tumor progression. Apolipoprotein E promotes lipid accumulation in tumor-associated macrophages (TAMs), contributing to the metabolic reprogramming of these cells (16). Lipid-associated macrophage subpopulations (LAM2) with high levels of APOE have also been detected in invasive breast cancer, correlating with immunosuppressive functions. This lipid dysregulation supports tumor growth and creates a pro-TME (17). These processes may vary with different alleles (Table 1); the ε4 allele exacerbates these effects by increasing the risk of tumor progression with or without cardiovascular comorbidities, while ε3 is often neutral, and ε2 may suppress tumor growth via enhanced anti-inflammatory lipid transport. For example, ε4 carriers show higher breast cancer susceptibility compared to ε3 (18).
| Alleles | Role in Breast Cancer | Role in CRCI | Key Mechanisms |
|---|---|---|---|
| ε2 | Potentially protective (reduced metastasis risk via anti-inflammatory effects and enhanced lipid clearance) | Lower susceptibility; better cognitive resilience, especially in older survivors post-chemotherapy | Reduced neuroinflammation, better cholesterol efflux |
| ε3 | Neutral/baseline; context-dependent modulation of tumor progression | Moderate risk; often used as reference for comparisons | Standard lipid transport, minimal disruption to TME or neurogenesis |
| ε4 | Pro-oncogenic (promotes M2 polarization, immunosuppression, increased cancer risk) | High risk; amplified neurotoxicity and long-term deficits | BBB dysfunction, oxidative stress, enhanced neuroinflammation |
Abbreviations: CRCI, cancer-related cognitive impairment; TME, tumor microenvironment; BBB, blood-brain barrier.
The APOE protein plays a complex, context-dependent role in changing immune responses within the TME (19). Apolipoprotein E can suppress immunity by stimulating regulatory T-cells and decreasing the activity of effector T-cells in several cancers (20). However, it can also promote immune cell infiltration under certain conditions, creating a more immunogenic environment (19). For example, in HER2-positive breast cancer, APOE’s immune-augmenting effects have been linked to higher levels of tumor-infiltrating lymphocytes (TILs), which may improve the effectiveness of immune checkpoint inhibitors like anti-PD-1/PD-L1 therapies (21). Because of this varied behavior, APOE could serve as both a biomarker and a therapeutic target to enhance immunotherapy outcomes in HER2-positive breast cancer. However, more research is needed to confirm its clinical potential (22).
3. Apolipoprotein E and Cognitive Decline in Breast Cancer Survivors
The CRCI, known as chemobrain, is an acute concern for 13% to 70% of breast cancer survivors. It influences cognitive functions, including memory, attention, processing speed, and executive function (23). In this context, the APOE ε4 allele has been introduced as a significant genetic risk factor, especially for subjects undergoing chemotherapy (10). Recent evidence has indicated that, in the long term, APOE ε4 carriers have worse cognitive function compared to non-carriers, with these deficits lasting for years after treatment. For example, in a study of breast cancer survivors averaging 8.8 years post-treatment, ε4 carriers showed notably lower scores in visual memory, spatial ability, and executive function (24).
These findings are supported by preclinical studies employing APOE knock-in mice treated with doxorubicin, a widely used chemotherapeutic agent. Mice carrying the APOE4 allele showed more severe impairments in spatial learning and greater reductions in gray matter volume in the frontal cortex compared to mice with the APOE3 allele (25). These findings provide critical mechanistic clues about how APOE may contribute to cognitive decline following chemotherapy. Further studies reveal that ε4 carriers without a smoking history showed reduced processing speed and working memory at 1, 6, and 18 months post-chemotherapy. Survivors aged 60 and above, who were exposed to chemotherapy and carried the APOE4 allele, showed notable declines in attention, processing speed, and executive function (26). These findings imply that APOE4 might intensify the effects of chemotherapy on brain aging, possibly hastening cognitive decline in this population.
Comparatively, APOE2 variations have been reported to have a protective role against cancer-related cognitive decline in older breast cancer survivors (27). Moreover, a lack of impairments in spatial memory in APOE3 mouse models treated with doxorubicin was observed compared with APOE4 ones (25, 28).
Briefly, the mechanisms underlying APOE4-associated CRCI involve several key pathways:
- Chemotherapy-induced neurotoxicity: APOE4 magnifies chemotherapy-induced neurotoxicity by mechanisms such as impaired hippocampal neurogenesis, oxidative damage, and neuroinflammation (10).
- Blood-brain barrier (BBB) dysfunction: APOE4 compromises BBB integrity, allowing neurotoxic chemotherapeutics like doxorubicin to penetrate the brain. This increased permeability contributes to neuronal damage and cognitive deficits (29).
- Neuroinflammation: APOE4 amplifies glial activation, leading to increased neuroinflammation and synaptic damage. This pro-inflammatory state exacerbates cognitive deficits in breast cancer survivors (30).
- Impaired hippocampal neurogenesis: APOE4 knock-in mice treated with doxorubicin exhibit reduced hippocampal neurogenesis, critical for learning and memory. This impairment is associated with spatial learning deficits and reduced frontal cortex gray matter volume (25, 28).
- Interactions with endocrine therapy: APOE4 may also interact with endocrine therapies, amplifying cognitive deficits in attention and learning domains, particularly in ε4 carriers (31, 32).
4. Shared Pathways: Cancer and Neurodegeneration
APOE4 links shared molecular pathways between cancer and neurodegenerative diseases (33). Oxidative stress and insulin resistance, for example, are common pathological factors in both breast cancer and Alzheimer’s disease (34). The mitochondrial dysfunction driven by APOE4 and its association with metabolic syndrome further underscores the convergence of these conditions (35). Identifying these related mechanisms may provide critical insights for developing new therapeutic strategies that simultaneously target cancer progression and cognitive decline.
5. Clinical Implications
Promoting tumor progression through immunosuppression in the TME and susceptibility to CRCI induced by APOE poses a therapeutic challenge, underscoring its function in targeted therapy of breast cancer and the need for medication therapy management. The clinical implications of the role of APOE require approaches that address personalized patient care in multiple ways. In this regard, genetic profiling could diagnose high-risk patients who are potentially at risk of cognitive destruction and allow for selecting personalized treatment modalities that consider oncological and cognitive outcomes. For example, breast cancer subjects carrying APOE ε4 may benefit from cognitive monitoring and neuroprotective interventions during chemotherapy (10). However, using genetic testing in routine oncology care and considering the efficacy of cognitive interventions requires ethical and practical considerations crucial to translating the multiple roles of APOE into applicable clinical programs.
6. Future Directions
Elucidating the molecular mechanisms by which APOE influences the TME and CRCI is critical to advancing our understanding and developing targeted therapies. Applied research should focus on molecules that target shared pathways between cancer and cognition, thereby bridging the gap between oncological and psychological outcomes. Investigating different APOE genotypes and their interactions with lifestyle factors in clinical studies to determine the extent of cancer risk and cognitive impairment, and the importance of response to treatment, could help optimize cancer therapies and reduce cognitive side effects, consequently improving both survival and quality of life for breast cancer survivors. In summary, future research should seamlessly integrate basic mechanistic studies, translational research, and clinical applications to explore the APOE function.
7. Conflicting Findings and Limitations
The ε4 isoform is consistently linked to increased tumor aggression and CRCI in many studies. However, conflicts exist; for instance, some longitudinal analyses find no worsening of cognition in ε4 carriers over time. Moreover, APOE’s role in immune augmentation in specific subtypes, such as HER2-positive, contradicts its immunosuppressive effects elsewhere. Also, there are some limitations in studies, including small cohort sizes, heterogeneous treatment protocols, ethnic biases in allele frequency data, and reliance on animal models that may not fully mimic human physiology. Larger, diverse trials are needed to resolve these inconsistencies.
8. Conclusions
The role of APOE in breast cancer development and CRCI highlights its potential for targeted therapy. In this regard, further research should clarify the allele-specific molecular mechanisms, focusing on the development of targeted therapies. Incorporating genetic profiling into clinical practice could help plan personalized treatment strategies that balance cancer treatment and preservation of cognitive function. As a result, clinicians can improve both tumor control and long-term mental health for survivors in the care of breast cancer patients.