1. Introduction
Henoch-Schonlein purpura (HSP) is a common form of vasculitis in children that affects multiple organs, including the skin, gastrointestinal system, and kidneys. This condition is typically marked by a distinctive rash, joint pain, abdominal discomfort, kidney inflammation, and swelling, especially in the hands and feet (1). While the rash usually resolves within 1 to 2 months, kidney and gastrointestinal symptoms can persist for a longer time (2). The incidence of HSP in children is reported to range from 3 to 26.7 cases per 1,000,000, although this figure may be an underestimation due to unreported cases (3). Renal involvement occurs in 30 to 50% of children with HSP, with boys more likely to experience renal issues between ages 5 to 8 years, while girls tend to be more affected between 4 to 10 years of age (4, 5).
Although the exact cause of HSP remains unclear, it is thought that a combination of factors such as infections, food or drug allergies, inflammatory conditions, and blood clotting disorders may play a role in its development (6). The most common sign of kidney involvement is microscopic hematuria, though macroscopic hematuria and proteinuria can also occur (1, 3, 6-9). Chronic kidney disease (CKD) may develop in 5 - 15% of children with HSP (7). Proteinuria is an important indicator of kidney damage, and persistent nephritic-range proteinuria can lead to kidney failure (8, 10). Kidney problems often begin within the first few weeks of the illness, so regular monitoring is essential to either restore normal kidney function or determine a new baseline. A considerable proportion of children with HSP develop renal involvement characterized by hematuria and proteinuria, commonly referred to as Henoch-Schonlein purpura nephritis (HSPN) (9). In severe cases, disease progression within the first few months after symptom onset may result in long-term complications (11). In more advanced stages of HSPN, renal function can deteriorate, leading to hypoproteinemia, hypertension, and persistent kidney impairment (10).
Identifying predictive factors is crucial to starting early steroid therapy for those at risk of significant organ involvement. Previous studies have explored the connection between factors like platelet (PLT) count, white blood cell (WBC) count, low lymphocyte counts, C-reactive protein (CRP) levels, mean platelet volume (MPV), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) with severe outcomes of HSP (10, 12, 13). The NLR, in particular, has shown promise as a cost-effective and reliable marker for inflammation and may serve as a predictive tool for hypertension in children with HSP and neutrophil-driven inflammation. However, no single laboratory marker has yet been identified as a reliable predictor of severe complications, such as renal involvement (11, 12), highlighting the need to assess whether combined or accessible markers can improve risk estimation. This study aims to evaluate easily calculable parameters like NLR, PLR, and MPV in HSP patients and compare these values between patients with and without renal involvement to explore their usefulness as indicators of renal symptoms.
2. Methods
2.1. Study Design and Setting
This cross-sectional study was conducted at affiliated hospitals of Zahedan University of Medical Sciences in Zahedan, Iran, from June 2019 to December 2020.
2.2. Study Population and Criteria
The study included children under 18 years of age who had been diagnosed with HSP and had complete medical and laboratory records. Children were excluded if they had autoimmune diseases, CKD, idiopathic nephrotic syndrome, use of medications before blood sampling, or missing laboratory data. After applying the eligibility criteria, 236 patients were included in the final analysis.
2.3. Diagnostic Criteria and Renal Involvement Definition
The diagnosis of HSP was confirmed according to the European League Against Rheumatism/Paediatric Rheumatology International Trials Organisation/Paediatric Rheumatology European Society (EULAR/PRINTO/PRES) criteria. These criteria require palpable non-thrombocytopenic purpura plus at least one of the following: Abdominal pain, IgA deposition on biopsy, arthritis/arthralgia, or renal involvement. Renal involvement was defined as the presence of either:
- Proteinuria: Protein level > 0.3 g/24 h or equivalent spot urine protein-to-creatinine ratio, and/or
- Hematuria: ≥ 5 red blood cells per high-power field on urine microscopy and/or dipstick result ≥ 2+.
Blood samples were obtained before initiation of any treatment.
2.4. Data Collection
Clinical and laboratory information was extracted from hospital records. Laboratory parameters included WBC count, neutrophil count, lymphocyte count, NLR, PLT count, PLR, hemoglobin, red blood cell indices [including mean corpuscular volume and mean corpuscular hemoglobin concentration (MCHC)], PLT indices [including MPV and platelet distribution width (PDW)], CRP, serum creatinine, and blood urea nitrogen. All analyses were performed in the Ali Ebn Abitalib Hospital laboratory using standard automated equipment.
2.5. Ethical Approval
Ethical approval was obtained from the institutional review board under reference number IR.IAU.ZAH.REC.1402.073. Written informed consent was obtained from all participants or their legal guardians.
2.6. Statistical Analysis
Data were analyzed using SPSS version 23.0 (SPSS, Inc., Chicago, IL, USA). Quantitative variables were presented as mean ± standard deviation, and comparisons between groups were performed using either the independent samples t-test or Mann-Whitney U test depending on data distribution. Qualitative variables were compared using the chi-square (χ²) test. Univariate and multivariate logistic regression analyses were performed to examine associations between laboratory variables and renal involvement. Multivariable models were adjusted for age and sex, which were available in medical records and considered clinically relevant potential confounders. A P-value ≤ 0.05 was considered statistically significant.
3. Results
This study evaluated clinical and laboratory factors associated with renal involvement in children diagnosed with HSP. Of the 236 patients included, 36.4% (n = 86) exhibited renal involvement. The mean age of all patients was 7.30 ± 3.18 years. Children with renal involvement were older on average (8.07 ± 3.51 years) than those without renal involvement (6.86 ± 2.90 years), and this difference was statistically significant in univariate analysis.
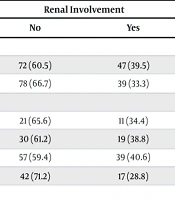
As shown in Table 1, gender distribution did not differ significantly between groups (P > 0.05). The proportion of renal involvement across seasons — including spring (34.4% of 32 spring diagnoses) — showed no significant variation (χ² = 1.989, P = 0.594). The length of hospital stay (< 5 days, 5–10 days, > 10 days) was also not associated with renal involvement (χ² = 2.816, P = 0.245).
| Variables/Status | Renal Involvement | Total | χ2 | P-Value | |
|---|---|---|---|---|---|
| No | Yes | ||||
| Gender | 0.967 | 0.235 | |||
| Girls | 72 (60.5) | 47 (39.5) | 119 (100) | ||
| Boys | 78 (66.7) | 39 (33.3) | 117 (100) | ||
| Seasons | 2.382 | 0.495 | |||
| Spring | 21 (65.6) | 11 (34.4) | 32 (100) | ||
| Summer | 30 (61.2) | 19 (38.8) | 49 (100) | ||
| Fall | 57 (59.4) | 39 (40.6) | 96 (100) | ||
| Winter | 42 (71.2) | 17 (28.8) | 59 (100) | ||
| Duration of hospital stay (d) | 3.338 | 0.189 | |||
| < 5 | 79 (68.7) | 36 (31.3) | 115 (100) | ||
| 5 - 10 | 61 (60.4) | 40 (39.6) | 101 (100) | ||
| > 10 | 10 (50) | 10 (50) | 20 (100) | ||
| Total | 150 (63.6) | 86 (36.4) | 236 (100) | ||
a Values are expressed as No. (%)
Table 2 presents the comparison of laboratory values between groups. White blood cell count was significantly higher in the renal involvement group (16,333.72 ± 5525.31/µL vs. 10,458.67 ± 3916.19/µL, P < 0.001). Similar patterns were observed for neutrophil percentage, NLR, and PLT count. In contrast, lymphocyte percentage and MPV were significantly lower in the renal involvement group (both P < 0.001). The magnitude of the mean WBC value in the renal group is high and may reflect acute inflammatory status; however, the unit and laboratory reference ranges should be interpreted with clinical caution.
| Lab Data/Renal Status | Mean ± SD | Mean Rank | Sum of Ranks | MWU | P-Value |
|---|---|---|---|---|---|
| WBC | 1819.5 | < 0.001 | |||
| No | 10,458.67 ± 3,916.19 | 87.63 | 13144.5 | ||
| Yes | 16,333.72 ± 5,525.31 | 172.34 | 14821.5 | ||
| Neutrophil | 1208.5 | < 0.001 | |||
| No | 59.14 ± 13.29 | 83.56 | 12533.5 | ||
| Yes | 80.94 ± 9.98 | 179.45 | 15432.5 | ||
| Lymphocyte | 1476 | < 0.001 | |||
| No | 38.44 ± 13.31 | 151.66 | 22749 | ||
| Yes | 18.96 ± 9.94 | 60.66 | 5217 | ||
| N/L ratio | 1767.5 | < 0.001 | |||
| No | 2.22 ± 2.24 | 87.28 | 13092.5 | ||
| Yes | 6.18 ± 4.74 | 172.95 | 14873.5 | ||
| P/L ratio | 6359.5 | 0.858 | |||
| No | 12.88 ± 10.11 | 117.9 | 17684.5 | ||
| Yes | 12.7 ± 8.21 | 119.55 | 10281.5 | ||
| Hb | 5879.5 | 0.258 | |||
| No | 11.72 ± 1.66 | 122.3 | 18345.5 | ||
| Yes | 11.47 ± 1.7 | 111.87 | 9620.5 | ||
| PLT | 4447.5 | < 0.001 | |||
| No | 346.79 ± 107.57 | 105.15 | 15772.5 | ||
| Yes | 420.2 ± 129.4 | 141.78 | 12193.5 | ||
| MPV | 2776 | < 0.001 | |||
| No | 8.6 ± 0.73 | 142.99 | 21449 | ||
| Yes | 7.81 ± 0.71 | 75.78 | 6517 | ||
| BS | 5948.5 | 0.32 | |||
| No | 98.33 ± 20.7 | 121.84 | 18276.5 | ||
| Yes | 98.38 ± 32.22 | 112.67 | 9689.5 | ||
| BUN | 6190 | 0.605 | |||
| No | 13.01 ± 6.87 | 116.77 | 17515 | ||
| Yes | 12.91 ± 5.4 | 121.52 | 10451 | ||
| Cr | 6061.5 | 0.423 | |||
| No | 0.56 ± 0.13 | 115.91 | 17386.5 | ||
| Yes | 0.6 ± 0.19 | 123.02 | 10579.5 | ||
| Na | 5661.5 | 0.117 | |||
| No | 138.59 ± 4.48 | 123.76 | 18563.5 | ||
| Yes | 138.07 ± 3.52 | 109.33 | 9402.5 | ||
| K | 6442 | 0.987 | |||
| No | 4.34 ± 0.39 | 118.45 | 17767 | ||
| Yes | 4.34 ± 0.49 | 118.59 | 10199 |
Abbreviations: WBC, white blood cell; N/L ratio, neutrophil-to-lymphocyte ratio; P/L ratio, platelet-to-lymphocyte ratio; PLT, platelet; MPV, mean platelet volume.
As shown in Table 3, univariate logistic regression demonstrated that age, WBC count, neutrophil percentage, lymphocyte percentage, NLR, and PLR were significantly associated with renal involvement (all P < 0.05). Although PLT count was significantly higher in group comparisons, it was not a significant predictor in regression analysis.
| Variables | Univariate | Multivariate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | S.E. | Wald | Sig. | Exp (B) | B | S.E. | Wald | Sig. | Exp (B) | |
| Sex (ref.: Boys) | 0.270 | 0.273 | 0.980 | 0.322 | 1.310 | 0.055 | 0.414 | 0.018 | 0.894 | 1.056 |
| Season (ref.: Spring) | ||||||||||
| Summer | 0.258 | 0.470 | 0.301 | 0.584 | 1.294 | 1.422 | 0.721 | 3.886 | .049 | 4.143 |
| Autumn | 0.361 | 0.413 | 0.764 | 0.382 | 1.435 | 0.180 | 0.624 | 0.083 | 0.773 | 1.197 |
| Winter | 0.482 | 0.355 | 1.838 | 0.175 | 1.619 | 0.431 | 0.524 | 0.676 | 0.411 | 1.539 |
| Age (y) | 0.115 | 0.044 | 6.967 | 0.008 | 1.122 | 0.079 | 0.067 | 1.375 | 0.241 | 1.082 |
| WBC | 0.000 | 0.000 | 41.206 | < 0.001 | 1.000 | 0.000 | 0.000 | 18.986 | < 0.001 | 1.000 |
| Seg | 0.052 | 0.010 | 25.241 | < 0.001 | 1.053 | 0.004 | 0.009 | 0.163 | 0.687 | 1.004 |
| Lymphocyte | -0.120 | 0.016 | 55.216 | < 0.001 | 0.887 | -0.111 | 0.031 | 13.213 | < 0.001 | 0.895 |
| N/L ratio | 0.463 | 0.072 | 41.098 | < 0.001 | 1.589 | -0.063 | 0.247 | 0.066 | 0.797 | 0.939 |
| P/L ratio | 0.078 | 0.014 | 30.223 | < 0.001 | 1.081 | 0.011 | 0.046 | 0.058 | 0.810 | 1.011 |
| Hb | -0.097 | 0.083 | 1.378 | 0.241 | 0.907 | -0.060 | 0.123 | 0.237 | 0.626 | 0.942 |
| PLT | -0.001 | 0.001 | 1.503 | 0.220 | 0.999 | -0.005 | 0.003 | 3.544 | 0.060 | 0.995 |
| MPV | -0.130 | 0.155 | 0.703 | 0.402 | 0.878 | -0.416 | 0.273 | 2.314 | 0.128 | 0.660 |
| BS | -0.003 | 0.006 | 0.332 | 0.564 | 0.997 | -0.004 | 0.008 | 0.212 | 0.646 | 0.996 |
| BUN | -0.008 | 0.022 | 0.127 | 0.721 | 0.992 | -0.053 | 0.040 | 1.793 | 0.181 | 0.948 |
| Cr | -0.094 | 0.445 | 0.045 | 0.832 | 0.910 | -2.264 | 1.461 | 2.402 | 0.121 | 0.104 |
| Na | -0.022 | 0.033 | 0.448 | 0.503 | 0.978 | -0.022 | 0.052 | 0.171 | 0.679 | 0.979 |
| K | 0.040 | 0.318 | 0.016 | 0.900 | 1.041 | -0.036 | 0.494 | 0.005 | 0.942 | 0.965 |
Abbreviations: WBC, white blood cell; N/L ratio, neutrophil-to-lymphocyte ratio; P/L ratio, platelet-to-lymphocyte ratio; PLT, platelet; MPV, mean platelet volume.
In the multivariate model, only WBC and lymphocyte percentage remained significant predictors of renal involvement (P < 0.001), while age, neutrophil percentage, NLR, and PLR no longer demonstrated independent effects. This shift highlights the influence of confounding among inflammatory markers and suggests that lymphocyte and WBC counts may serve as more robust predictors than composite ratios such as NLR or PLR.
4. Discussion
This study retrospectively examined demographic and laboratory data to identify risk factors for renal involvement in children with HSP. The results showed that 36.4% of the patients experienced kidney involvement, with a notably higher incidence in girls compared to boys. Univariate logistic regression analysis revealed that factors such as WBC count, neutrophil count, lymphocyte count, NLR, and PLR were significantly associated with renal involvement in children with HSP. However, some of these factors lost their significance in the multivariate analysis. Additionally, the MPV was found to be lower in children with renal involvement.
Interestingly, the study also showed that the risk factors of sex and age of onset for renal involvement in pediatric HSP presented some differences. Children with kidney involvement were generally older, and while 33.3% of boys had renal issues, around 39.5% of girls experienced similar problems. These findings align with a recent study by Sadeghi-Bojd et al. (14), which found that approximately 59% of children with HSP had kidney complications. Similarly, Xi et al. (6) conducted a study involving 146 patients, 96 of whom had HSP and 50 with HSPN, reporting that 34.2% of HSP patients experienced kidney problems. Previous studies have shown varying rates of renal issues in children with HSP, ranging from 30% to 50%, with a small percentage progressing to end-stage renal disease (11).
Kim et al. (4) explored that 36.0% of HSP children had kidney findings, similar to our and other studies, which included outcomes from microscopic hematuria to severe proteinuria. While HSPN is usually mild and temporary, it can sometimes lead to long-term kidney findings (15). In Sadeghi-Bojd et al.'s recent study (14), it was found that the average age of patients at diagnosis was 7.37 ± 3.19 years, with most being females (51.13%). Kilic and Demir (16) and Ekinci et al. (2) also noted that the majority of patients were over 7 years old. Sano et al. (17) suggested that being older than 4 years was a risk factor for kidney problems in patients. Kilic and Demir (16) and Ekinci et al. (2) also discovered that boys were more prevalent, which contrasts with the findings of the present study and a recent study by Sadeghi-Bojd et al. (14) that reported a higher proportion of girls in HSP patients. Gomez (18) reported a nearly equal sex ratio of about 1:1 among patients, which closely matches our findings, though they observed the average age of onset to be 7.02 years. Wang et al. (19) found renal involvement in 35.6% of HSP patients, with 4.39% experiencing severe kidney disease. Several factors were identified as significant in influencing renal involvement, including an age of over 6 years at the time of onset, colder seasons, and a delay of more than 8 days between the appearance of symptoms.
In our study, children with HSP and renal involvement had higher levels of WBCs, neutrophils, NLR, and PLTs. In contrast, their lymphocyte count and MPV were lower. Interestingly, the PLR did not show significant differences between the groups of children with and without kidney involvement.
Kim et al. (5) found similar trends, showing that children with renal involvement had higher neutrophil-to-lymphocyte and PLR s compared to those without kidney issues. They also observed no significant differences in hemoglobin levels, PLT counts, joint pain, or gastrointestinal symptoms between the two groups. In another study by Ozdemir et al. (20), a comparative analysis of various hematologic parameters revealed that patients with internal organ involvement, including kidney and gastrointestinal issues, had elevated levels of leukocytes, neutrophils, and monocytes. They also had higher NLRs and CRP levels. However, there were no significant differences in lymphocyte counts, PLT counts, or the PLR between the groups.
In a study conducted by Xi et al. (6), which encompassed 146 HSP patients, findings revealed that 34.2% of them presented with renal involvement. The investigation further highlighted an inverse relationship between CRP and NLR in correlation to renal complications in pediatric HSP cases. Through univariate logistic regression analysis, it was established that variables such as age, WBC count, PDW, MCHC, CRP, and NLR emerged as noteworthy independent indicators for renal involvement. Moreover, age, PDW, CRP, and NLR were recognized as significant factors through multivariate logistic regression analysis. Similarly, a study by Kim et al. (4) found that logistic regression analysis identified female gender and the NLR as significant risk factors for renal complications in HSP. Their research also highlighted the role of WBC count, PDW, MCHC, CRP, NLR, and immunoglobulin G (IgG) as valuable, independent predictors of kidney involvement. Multivariate logistic regression analysis confirmed the importance of PDW, CRP, NLR, and IgG in predicting renal complications in HSP patients.
However, the study has some limitations that must be considered. The retrospective design and the focus on children from a single center could limit the generalizability of the findings to a wider population. Additionally, the study only examined laboratory values during the acute phase of the disease, which means changes in laboratory data before, during, and after renal involvement were not fully captured. These factors underscore the need for more comprehensive, longitudinal studies to gain a deeper understanding of the disease's progression and its effects on laboratory values over time.
4.1. Conclusions
In conclusion, the study found notable changes in laboratory values among children with HSP who had renal involvement compared to those without kidney complications. Specifically, there was an increase in WBC count, neutrophil count, NLR, and PLT count, while MPV and lymphocyte count were decreased. To effectively detect kidney damage in children with HSP, it is crucial to conduct a complete blood count (CBC) upon admission or on the first day of hospitalization. This test can serve as an important tool in identifying renal complications early in the course of the disease.