1. Background
Interventional options are being considered for pain relief when conservative management is not efficacious, and pharmacological options have undesirable side effects (1, 2). Pulsed radiofrequency (PRF) procedure has demonstrated clinical effectiveness, significantly reducing pain of different etiology and localization, such as chronic lumbar radicular pain due to herniated disk, failed back surgery syndrome, and postamputation phantom pain (3-5).
PRF inhibits oxidative stress and restores antioxidant enzymes by decreasing inflammatory markers in muscles (6). PRF target are small diameter axons consisting of the C and A delta fibers, as evidenced by the lack of activating transcription factor 3 (ATF3) upregulation-common marker for cellular stress. Analgesic action of PRF enhances inhibition of noradrenergic and serotonergic descending pain pathways, which was demonstrated in an adjuvant induced inflammatory pain model in rats (7, 8).
Matrix metalloproteinases (MMP) are enzymes that catalyze extracellular matrix degradation. MMP-2 has also been shown to be involved in painful conditions. This up-regulation of MMP-2 was also observed in both the dorsal root ganglion (DRG) and spinal cord in response to spinal nerve ligation. Treatment with MMP-2 inhibitors attenuates the allodynic response observed after the above-mentioned procedure (9).
Nestin (Nes) is a type VI intermediate filament protein. Nestin is an acronym for neuroectodermal stem cell intermediary filament. This process is regulated by the phosphorylation of nestin at its threonine residues by kinase cdc2 (10).
Radicular pain is caused by irritation of a DRG, which forms a very significant part of acute nociception, as well as in induction and maintenance of chronic pain (7, 11).
2. Methods
2.1. General Conditions
The study protocol was approved by the Animal Care Ethics Committee, Riga, Latvia (Nr. 41, 2012.26.01). In this study, we used seven clinically healthy adult female domestic pigs weighing 59-65 kg. The animals were housed in a standard farm in Jelgava district, Latvia, with access to food and water ad libitum.
2.2. Pulsed Radiofrequency Procedure
All efforts were made to treat the test subjects humanely to reduce anxiety and stress. Prior to inducing anesthesia, premedication was administered intramuscularly with ketamine (10 mg/kg), azaperon (4 mg/kg), and atropine (0.02 mg/kg). After pre-oxygenation, intravenous induction was carried out with pentobarbital (6 mg/kg), and the pigs were placed in the prone position following endotracheal intubation. Monitoring included an electrocardiogram (ECG), non-invasive blood pressure, pulse oximetry, and temperature. Anesthesia was maintained using inhalation of Isoflurane (2 Vol %). Under fluoroscopy imaging guidance (C-arm, Philips), 22G radiofrequency cannulas (10 cm length, 5 mm active tip RF cannula, S-1005, Neurotherm) were introduced transforaminally to approach the DRG in an optimal position. Motor stimulation (2Hz) was positive between 0.5V - 0.8V, as observed by the contraction of appropriate leg muscles. The temperature was maintained so as not to exceed 42°C during the procedure in all cases, and impedance was closely monitored (180 – 220 Ω). Lumbar DRGs at four levels (L1, L2, L3, L4) were closely affected. The DRGs on the appropriate contralateral side were used as controls.
2.3. Observation of the Animals and Tissue Preparation
Animals were checked daily for their general condition and for any potential complications. Thirty days after the procedure, the animals were euthanized using intravenous Pentobarbital (200 mg/kg). The lumbar part of the vertebrae was separated and placed in a 10% buffer of formaldehyde for 30 days. After tissue fixations on both sides of the spine from T12 - L5 using a hemilaminectomy method, the corresponding DRGs were prepared for immunohistochemical and histopathological analysis. They were embedded in paraffin and then sliced by microtome to obtain 3-μm thick slide sections.
2.4. Immunohistochemistry
To analyze the expression of nestin and MMP-2, multiple 3μm-thick sections of the paraffin-embedded DRGs were examined for immunohistochemistry. The tissue slides were heated at 60°C for 30 - 60 min. The tissues were deparaffinized and rinsed in distilled water (H2O) for 5 min. Antigen retrieval was done by microwaving in 0.1 mol/L sodium citrate (pH 6.0) for 5 min. The primary antibodies utilized in immunohistochemistry were goat anti-human matrix metallopeptidase 2 (MMP 2, code-Nr. DUB 03, dilution 1:100) and rabbit anti-human nestin (Nes, code-Nr. AB 5968, dilution 1:250, Abcam, United Kingdom). Finally, hematoxylin and eosin (H&E) staining was performed on each sample. Sections were covered with a polystyrene-based medium, and a coverslip was applied.
2.5. Analysis
For quantitative analysis, we counted the cells with biomarker expression, using light microscopy in three fields of vision with x400 magnification. Counting was done by a blinded and experienced investigator. The mean ± SD was used for quantitative data. For statistical analysis, we used Student’s t-test to compare mean values in the two groups. A P-value less than 0.05 was considered as statistically significant.
2.6. Data Distribution Rule
The measured values were the cell counts of a definite type per field of vision under the microscope. The specimen area considerably exceeded the field of vision, so the probability of a separate cell getting into the field would be low, although the cell count in the specimen was high. Therefore, it could be assumed that the cell count per field complies with the Poisson distribution. If the mathematical expectation for the Poisson distribution is 10 or greater, the mathematical expectation for the normal distribution can be approximated. The use of the normal distribution to describe data allows the use of well-known parametric statistics methods, such as the Student t-test and dispersion analysis. The probability diagram, as well as the Kolmogorov and Pearson criteria were used to determine the normality of the data.
3. Results
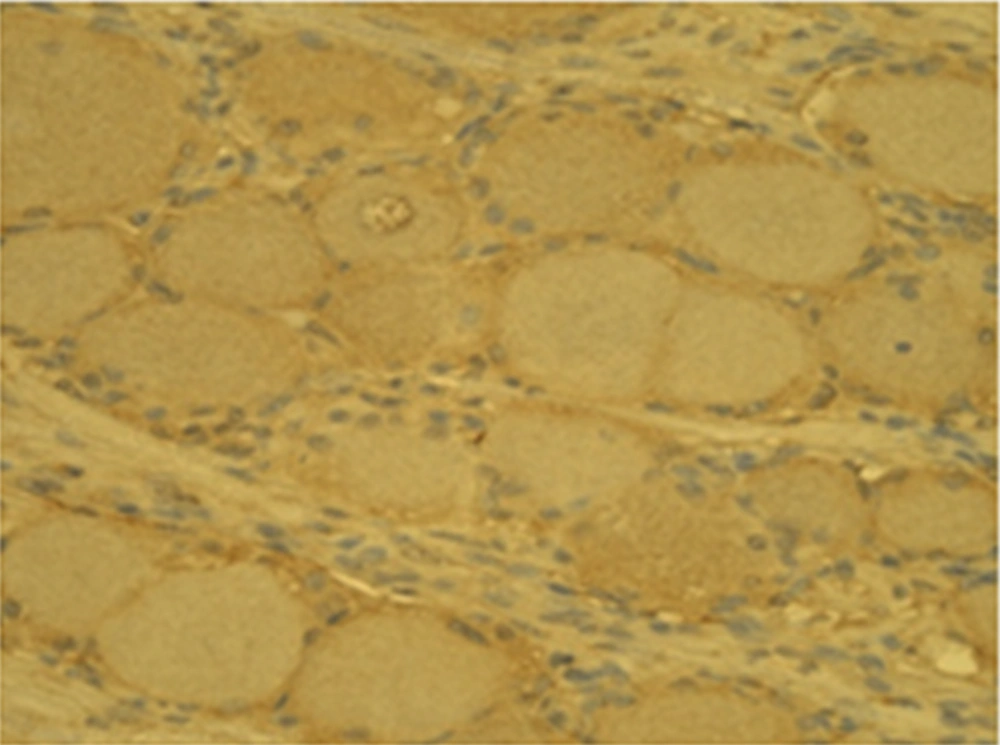
PRF-DRG increases the expression of biomarkers nestin and MMP-2. The number of cells with nestin and MMP-2 expression were larger on the PRF side compared to the control side.
Generally, the number of MMP-2 containing gangliocytes was higher on the PRF side (26.2 ± 3.2) than on the control side (14.1 ± 2.3) (Figures 1 and 2).
The same relation was also observed for nestin positive gangliocytes on the experimental side (28.4 ± 3.3) and on the control side (16.1 ± 3.4), respectively (Figures 3 and 4).
In our study, glial cells in the spinal ganglia on both sides also showed immuno-reactivity. The summary of the data is given in Table 1. Separate specimen data is summarized in Table 2. Probability diagrams were plotted for each of the factors (MMP-2, nestin) (Figure 5). The diagrams obtained after omitting terminal points are close enough to a straight line, which corresponds to a normal distribution. The Kolmogorov test was used to determine the normality of the cell count distribution in each of the specimen groups, with a significance level of 0.05. These results are summarized in Table 3. The performed tests confirm that the hypothesis of normal cell count distribution in the specimen could be used for further calculations. The two-sided Student’s t-test associated with the null-hypothesis. In other words, the study procedure did not influence MMP-2 factor expression in ganglion cells (t = 5.8), while the number of degrees of freedom was 26 (number of measurements - 1) (P-value = 3.9 × 10-6).
| Factors | Control | Experimental | P-Value |
|---|---|---|---|
| MMP-2 | 14.1 ± 2.3 | 26.2 ± 3.2 | < 0.05 |
| Nestin | 16.1 ± 3.4 | 28.4 ± 3.3 | < 0.05 |
| Vertebra | MMP-2 | Nestin | ||
|---|---|---|---|---|
| Control | Study | Control | Study | |
| I Ildze | ||||
| L1 | 13 | 26 | 22 | 37 |
| L2 | 16 | 28 | 20 | 29 |
| L3 | 22 | 36 | 16 | 26 |
| II Lavize | ||||
| L1 | 10 | 26 | 17 | 28 |
| L2 | 15 | 27 | 21 | 36 |
| L3 | 15 | 24 | 21 | 34 |
| L4 | 14 | 23 | 18 | 29 |
| III Luize | ||||
| L1 | 16 | 31 | 18 | 29 |
| L2 | 13 | 25 | 19 | 30 |
| L3 | 13 | 23 | 20 | 29 |
| L4 | 14 | 30 | 18 | 27 |
| IV Laila | ||||
| L1 | 13 | 23 | 19 | 31 |
| L2 | 17 | 29 | 20 | 28 |
| L3 | 14 | 23 | 14 | 29 |
| L4 | 11 | 23 | 14 | 25 |
| V Laura | ||||
| L1 | 16 | 27 | 16 | 28 |
| L2 | 13 | 27 | 18 | 30 |
| L3 | 14 | 25 | 14 | 26 |
| L4 | 14 | 26 | 18 | 27 |
| L1 | 12 | 23 | 11 | 26 |
| Specimen Group | Kolmogorov Statistics λ | Critical Value at α = 0.05 λkrit | Data Conformity with the Hypothesis of Normal Distribution |
|---|---|---|---|
| Control MMP-2 | 0.549 | 1.35 | λ < λkrit hypothesis is true |
| Study MMP-2 | 0.405 | 1.35 | λ < λkrit hypothesis is true |
| Study nesten | 0.449 | 1.35 | λ < λkrit hypothesis is true |
This significant P-value allows us to claim that the study procedure increased MMP-2 factor expression. The two-sided Student’s t-test was associated with the null-hypothesis, meaning that the study procedure did not influence nestin expression in ganglion cells (t = 4.56; P-value = 1.1 × 10-4). This P-value allows us to safely claim that the PRF study procedure increased nestin factor expression.
4. Discussion
The aim of our study was to evaluate the expression of nestin and MMP-2 biomarkers in gangliocytes of the DRG in domestic pigs after PRF.
In our previous study, we analyzed the histological effects of PRF on the DRGs of domestic pigs and evaluated the expression of biomarkers (neurofilaments (NF), glial fibrillary acidic protein, and heat shock protein - 70). We concluded that the increase in neural tissue cytoskeleton and cell stress factors in gangliocytes after PRF suggested cellular activation, regeneration processes, and inhibitors’ significancy due to the reduction of oxidative stress (7).
The results of our study implied that the PRF treatment of porcine DRG results in a statistically significant increase in the number of gangliocytes expressing MMP-2 and nestin. This is the first report about nestin and MMP-2 study on neuronal tissue after the application of PRF. The result from this original study is promising due to the clinical application of neural tissue regeneration and proliferation. Importantly, these two cellular markers share a multitude of common features. Nestin is implicated in a variety of normal and pathological conditions associated with active cellular turnover. We also observed that PRF application in the lumbar spinal gangliocytes increased neural tissue cytoskeleton factors like neurofilaments (NF) (7).
Likewise, increased nestin expression has also been reported in areas of tissue regeneration: for instance, in cardiac tissue, nestin (+) resident cells participate in post-ischemic revascularization; in the brain, nestin expression can be induced in post-mitotic astrocytes by an ischemic insult, resulting in gliosis; and in the inner ear, an increase in the number of nestin-positive cochlear cells is observed in response to a noise-induced lesion. In addition, increased nestin expression was observed in the major pelvic ganglion of rats after crush injury of the cavernous nerve, in the dorsal horn of mice after L5 spinal nerve transection, and in the murine ciliary body following damage to the optic nerve (12).
Furthermore, nestin is constitutively expressed in adult cells exhibiting progenitor properties, such as neural hippocampal stem cells, bone marrow stem cells, and satellite glial cells of the DRG. Thus, nestin can be viewed as a marker of cellular immaturity, potential, and capacity for self-renewal. Both nestin and MMP-2 are abundantly expressed during embryonal development, while in the postnatal period, these markers appear to be upregulated in stem cells and progenitor cells (13). In addition, both nestin and MMP-2 are upregulated in the DRG following mechanical injury of a corresponding peripheral nerve (14).
Finally, expression of nestin, as well as of MMP-2, is increased in regenerating tissue (15). Therefore, it can be assumed that nestin and MMP-2 are markers which reflect relative cellular immaturity, increased capacity of self-renewal, high proliferation rates, and augmented mobility. Whether induction of these properties in gangliocytes by application of PRF to the DRG is beneficial or not remains to be evaluated (9).
In addition, local alterations in DRG cells, exemplified by modified expression of proinflammatory enzymes, ion channels, micro-RNAs, membrane receptors, and growth factors, have been shown to induce and maintain neuropathic pain (16).
Since both nestin and MMP-2 levels have been shown to increase in response to a variety of noxious insults, PRF can be viewed as a novel artificial stimulus, which works by triggering the relatively nonspecific cellular stress response characterized by increased nestin and MMP-2 expression (17).
In conclusion, the increase in the number of MMP-2-containing gangliocytes one month after the PRF procedure in the lumbar DRG demonstrated active gangliocyte degeneration followed by the replacement of neural cells. PRF in spinal gangliocytes of the lumbar region increases Nes factor expression, indicating neural remodeling and regeneration. The clinical application of these findings should be evaluated in future studies, which may illustrate some action mechanisms of PRF.