1. Background
Neuropathic pain is a clinical condition in which the patient experiences unpleasant sensations or pain in response to normally innocuous sensory input. In this case, the pain is either continuous or occur in bursts. Compelling evidence suggests that neuropathic pain is caused by neuroplastic changes and other pathogenic processes in both central and peripheral structures (1-4). About 600,000 new chronic pain cases are annually diagnosed in the United States, and 75 - 80 million Americans suffer from neuropathic pain.
The mismatch between subjective pain severity and objective stimulation makes neuropathic pain particularly distressing (1). Furthermore, the separate or combined use of the existing therapies, including non-steroidal anti-inflammatory drugs (NSAIDs), narcotics, anticonvulsants, antidepressants, and local anesthetics, has provided no satisfactory results in many cases (2, 3). Some intrathecal medications (e.g., amitriptyline, doxepin, meperidine, morphine, dexmedetomidine, fentanyl, and bupivacaine) have been used to treat the pain (4-7). In this regard, dextromethorphan oral is used as a pain killer as well (8). The intractability of many cases of neuropathic pain is undoubtedly because of pathogenic processes, including currently unknown mechanisms, not targeted by the existing drugs. Nonetheless, the dysregulated levels of numerous signaling factors (and/or receptors), including elevated glutamate, P-substance, neurokinin, decreased endogenous opioids, and increased glutamate sensitivity of N-methyl-D-aspartate receptors (NMDARs), are strongly implicated in neuropathic pain (3, 9, 10). Elevated glutamate release, the ensuing hyperactivity of NMDARs, and the downstream activation of calcium-dependent kinase pathways may promote nociceptive transmission, thereby leading to hyperalgesia (11-13). These features may contribute to central pain sensitization and neuropathic pain by enhancing neuroplasticity (14, 15). In contrast, NMDA antagonists preventing calcium influx in the presence of glutamate may prevent neuroplastic processes underlying neuropathic pain (16-18). In particular, the blockade of NMDA receptors in the spinal cord may be an effective strategy to slow down or prevent the development of neuropathic pain (19, 20). Dextromethorphan is one of the non-competitive NMDA receptor antagonists (21).
Peripheral glucocorticoid receptors (GRs) are altered by peripheral nerve injury and thus may modulate the development of neuropathic pain (22). Moreover, glucocorticoids are the critical regulators of neuroinflammation, a major driver of nociceptive signaling (23), which are aroused following the nervous system’s injury. This is while the effectiveness of GR signaling in neuropathic pain has not been widely examined (24, 25).
This study examined the efficacy of intrathecal dextromethorphan (iDMP), a non-competitive NMDA receptor antagonist clinically used as a non-opioid antitussive agent (21, 26), on experimental hyperalgesia and the GR expression in the spinal cord (27, 28).
2. Objectives
This study aimed to evaluate the effect of the non-competitive NMDAR antagonist dextromethorphan on partial sciatic nerve ligation (PSL)-induced neuropathic pain and the spinal expression of the glucocorticoid receptor (GR).
3. Methods
3.1. Study Design and Animal Selection
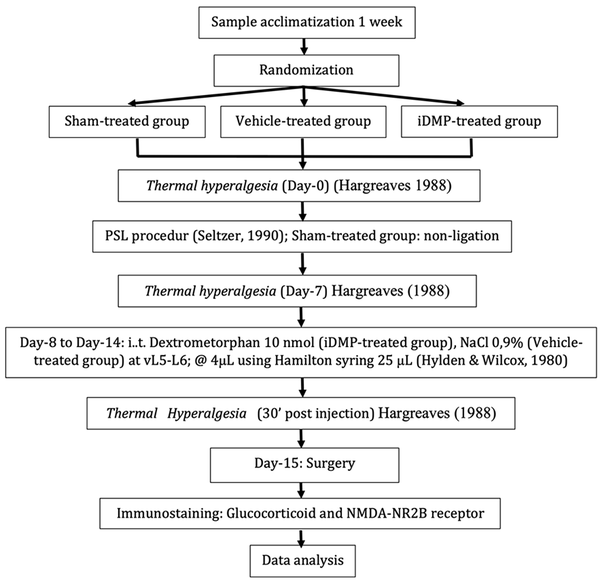
The manuscript was described according to the ARRIVE guideline. The flow diagram of the research procedures is illustrated in Figure 1.
The Animal Care and Use Committee affiliated to the Faculty of Veterinary Medicine, Airlangga University, approved this project (no.: 106-KE). Then 18 male mice (2 - 3 months old, 25 - 30 g) were obtained from the Faculty of Veterinary Medicine, Airlangga University, and housed in separate cages at a controlled temperature (20°C - 25°C) for seven days, while they had free access to food and water before the experiments.
3.2. Groups and Drug Administration
The sample size replication was estimated using the Federer formula for hypothesis-testing with a correction factor of 10%. The previously-reported mean difference between the groups was 0.46. Z-scores of 1.645 and 1.24 were considered for α = 5% and β= 10%. According to the aforementioned formula, six replications were estimated to be required in each group. The mice were randomly assigned into three groups (namely a sham group, a vehicle-treated control group, and an iDMP-treated group). Both vehicle and iDMP groups were subjected to PSL using the Seltzer procedure described below; however, the sciatic nerve was exposed to no ligation in the sham group (27, 29). The prepared drugs (10 nmol DMP (Bernofarm Pharmaceutical Company, Surabaya Indonesia) in 0.9% saline) were administered intrathecally between the 5th and 6th lumbar vertebrae once a day from day 8 to 14 following the PSL surgery.
3.3. Experimental Neuropathic Pain-Induction
Partial sciatic nerve ligation was performed in 12 randomly selected mice according to the Seltzer procedure under ether anesthesia. Briefly, the partial cross-section of the left sciatic nerve (~1/3 - 1/2 of the total thickness) was ligated at a high-thigh level using an 8/0 nylon monofilament suture. The sutured nerve was then placed back, and the overlying muscles and skin were sutured using silk 3/0 (27, 29). All mice were housed in separated cages for 2 hours during the recovery period. The sham surgery group (n = 6) received the same procedures; however, the nerve was not ligated.
3.4. Thermal Hyperalgesia Assessment
Thermal hyperalgesia was measured using a stainless-steel heating plate at 48°C. The onset of hindpaw shaking, hindpaw licking, or jumping in response to heating plate activation was recorded three times at 15-min intervals and then averaged (14). Hyperalgesia was defined as a more rapid response than the presurgical baseline. To avoid fatal injury, the maximum test duration was 30 s (28). Thermal responses were measured one day before the PSL procedure (baseline), on days 1, 3, 5, and 7 after surgery (drug-free period), and again 30 min after the saline or iDMP administration on days 8 - 14 after surgery (treatment period).
3.5. Histology Preparation and Staining
On day 15 after surgery, the mice were sacrificed by decapitation under ether anesthesia. Spinal cord sampling was performed by inserting an 18-gauge Surflo® catheter (Terumo Corporation, Tokyo, Japan) into the cranial end of the vertebral column and flushed with sterile water. The whole section of the spinal cord tissue was then fixed with 10% paraformaldehyde in phosphate-buffered saline (PBS) for 12 - 18 hours. The fixed tissue was embedded in paraffin (Thermo Scientific Histoclast, UK), sectioned at 4-µm thickness using a cryostat with a Leica 819 microtome blade on a Leica RM2235 microtome (Leica microsystem Nussloch GmbH, Nussloch, Germany), and collected on the labeled poly-L-lysine-coated glass slides. The slides were dried at room temperature and then processed for hematoxylin and eosin (HE) staining (Merck, Germany) and immunohistochemistry.
The slides were first placed on a warm plate at 56°C - 62°C for 30 min (HE) or 1 h (immunostaining), deparaffinized in xylene (for 5 min), rehydrated in degraded ethanol (99%, 96%, 90%, 80%, and 70%) for 5 min (HE) or 2 min (immunostaining) at each concentration, washed with tap water, and then rinsed in deionized (DI) water.
For HE staining, the processed slides were incubated in Mayer’s hematoxylin solution for 5 - 10 min, washed three times in DI, treated for 30 - 120 s using four drops of eosin solution, dehydrated in graded ethanol (70%, 80%, 90%, 96%, and 100% for 5 min each), cleared after being washed three times with xylene, and sealed in coverslips. For immunostaining, the processed slides were first treated with 200 µL of 3% H2O2 and 200 mL methanol for 5 min to block endogenous peroxidase activity, washed with DI water, and heated for 10 min by warmed citrate buffer for antigen retrieval. After being cooled down in citrate buffer for 20 min, the slides were washed with DI water and then with 1× PBS. The slides were wiped carefully on the outside of tissue edge, and the target area was carefully demarcated with a super PAP Pen. The slides were then drained, treated with four drops of block serum-free protein (Background Sniper) for 5 min, washed with 1× PBS, and incubated in 200 µL Tris buffer containing 1 µL of GR/NR3C1 antibody (rabbit polyclonal; 1:100; NB100-92252) within a magnetic immunostaining device for 1 h. The slides were then washed with 1× PBS, treated with Trekkie Universal Link (4 drops) for 10 min at room temperature, and wiped carefully on the outside of pen marked area. Next, the slides were rewashed with 1× PBS and treated with four drops of TrekAvidin-HRP for 10 min, and then with 1 mL of Betazoid DAB Chromogen solution and 1 drop of DAB Chromogen. Afterward, the slides were washed with tap water and treated with four drops of Meyer’s hematoxylin for 2 min, dehydrated in graded ethanol (70%, 80%, 90%, 96%, and 100%) for 2 min per concentration, cleared after being washed three times in xylene, and sealed with coverslips.
The full stained sections were viewed under an Olympus BX51 U-MDOBE light microscope (Olympus Corporation, Tokyo, Japan) at 100× and 400× magnification to assess the spinal cord histology and identify individual immunostained neurons and glial cells. Moreover, the GR expressions in the ipsilateral dorsal horn of the spinal cord were counted for each animal.
3.6. Statistical Analysis
All values are expressed as mean ± standard deviation. Means of thermal withdrawal on specific observation days (before or after PSL) were compared within groups by repeated-measure ANOVA using Bonferroni correction post-hoc analysis for each time-point measurement. P < 0.004 (two-tailed) was set as the significance level of the test. Moreover, the means of the GR expressions were compared using one-way ANOVA and LSD post-hoc tests to have pair-wise comparisons in homogenic-variance datasets according to the Levene’s test (P > 0.005). However, the means of other datasets with non-homogenic variance (Levene’s test; P < 0.05) were compared by the Brown-Forsythe’s test and post-hoc Games-Howell test. P < 0.05 (two-tailed) as set as the significance level for receptor expression tests. The statistical analyses were conducted using IBM SPSS® software version 20 for Windows (IBM Corp., Armonk, N.Y., USA).
4. Results
4.1. PSL-Induced Thermal Hypersensitivity
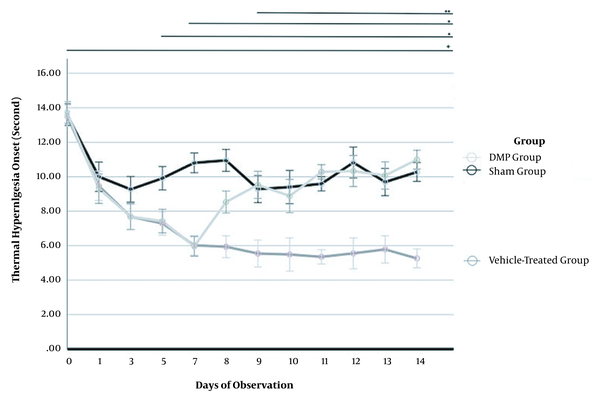
The thermal withdrawal response at baseline (before PSL) revealed no difference among the mouse groups on baseline (P = 0.828) and Day 1 (P = 0.643). However, shortening of time responses during hotplate stimulation was started on day 3 after PSL (P = 0.045) (Figure 2). Accordingly, PSL successfully induced thermal hyperalgesia as a neuropathic pain model.
Induction of thermal hyperalgesia by partial sciatic nerve ligation (PSL) and mitigation by intrathecal dextromethorphan (iDMP) in mice. Thermal sensitivity was measured by the withdrawal time to hotplate stimulation before PSL, after PSL, and before treatment (days 0 - 7), and during treatment with saline vehicle or iDMP (days 8 - 14). Compared to baseline, all time points showed a significant difference (+, P < 0.001). A, Significant hyperalgesia (shorter withdrawal time) was observed on days 5 and 7 (*, P < 0.001); B, This thermal hyperalgesia was partially abrogated after the first iDMP treatment and completely suppressed by subsequent injections on days 9 - 14 (P < 0.001** vs. vehicle group).
4.2. iDMP-Suppressed Thermal Hyperalgesia
The overall thermal withdrawal response for each time point revealed a significant difference (P < 0.001), compared to the baseline. The thermal withdrawal response was significantly slower (hyperalgesia lower) after the first administration of iDMP on Day 8 after PSL in the iDMP group, compared to the vehicle-treated PSL group (P < 0.001). Further, the mean response of the iDMP group did not differ significantly from that of the sham group from days 9 to 14, indicating the complete suppression of PSL-dependent hyperalgesia. Alternatively, the vehicle-treated group demonstrated thermal hyperalgesia on all treatment days (8 - 14), compared to the sham and iDMP groups (P < 0.001), indicating that iDMP can inhibit thermal hyperalgesia induced by PSL (Figure 2 and Table 1).
| Time | Number | Onset Thermal Hyperalgesia (Second) | ||
|---|---|---|---|---|
| Sham | Vehicle-Treated | iDMP | ||
| Day 7 | 6 | 10,81 ± 0,86 | 6,02 ± 0,39 | 5,96 ± 0,64 |
| Day 8 | 6 | 10,94 ± 0,92 | 5,94 ± 0,39 | 8,53 ± 0,79 |
| Day 9 | 6 | 9,28 ± 0,73 | 5,54 ± 0,31 | 9,53 ± 1,35 |
| Day 10 | 6 | 9,40 ± 0,57 | 5,49 ± 0,41 | 8,88 ± 1,79 |
| Day 11 | 6 | 9,59 ± 0,41 | 5,35 ± 0,28 | 10,28 ± 0,65 |
| Day 12 | 6 | 10,88 ± 0,56 | 5,56 ± 0,71 | 10,33 ± 1,55 |
| Day 13 | 6 | 9,61 ± 1,00 | 5,78 ± 0,68 | 10,07 ± 1,03 |
| Day 14 | 6 | 10,34 ± 0,57 | 5,26 ± 0,73 | 10,99 ± 0,61 |
| P-value | < 0,05 | > 0,05 | < 0,05 | |
Onset Thermal Hyperalgesia Time on Warm Plate
4.3. iDMP-Suppressed PSL-Induced Upregulation of Glucocorticoid Receptors in Spinal Cord
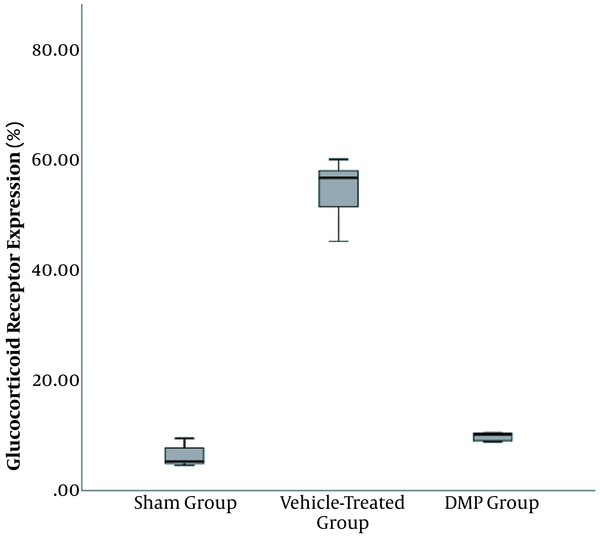
The GR expression of the spinal cord differed significantly among the treatment groups (P < 0.001). Using the LSD test, the post-hoc analysis was significantly higher in the vehicle-treated group, compared to the iDMP (P < 0.001) and sham groups (P < 0.001). The GR expression was significantly higher in the vehicle-treated group (55.64 ± 4.50), compared to other groups (P < 0.001). The iDMP group (9.99 ± 0.66) showed significantly higher GR expression compared to the sham group (6.30 ± 1.96) (P = 0.043) (Figures 3 and 4).
GR expression quantification in ipsilateral dorsal spinal cord. The GR expression was significantly higher in the vehicle-treated group (55.64 ± 4.50), compared to the other groups (P < 0.001). The iDMP group (9.99 ± 0.66) showed a slightly significant difference from the sham group (6.30 ± 1.96) (P = 0.043).
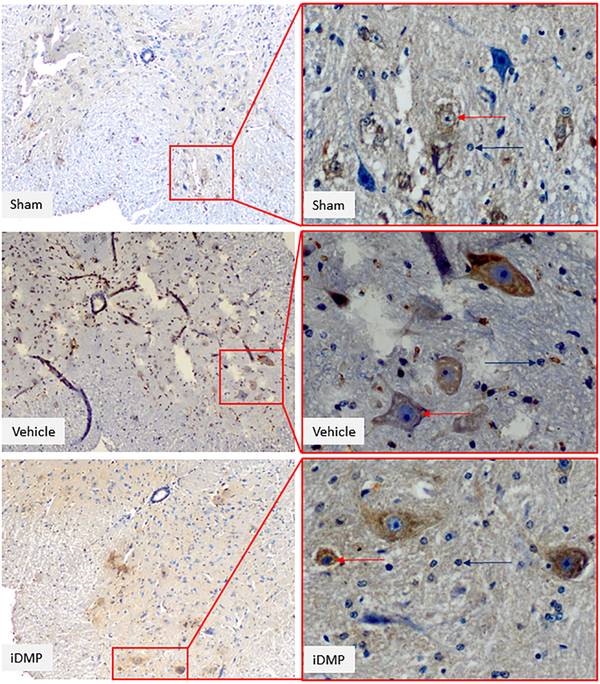
Glucocorticoid immunostaining in sham, vehicle-treated, and iDMP-treated groups Spinal cord sections were retrieved on day 15 following the PSL surgery. Immunostaining of ipsilateral spinal dorsal horn sections at 100× and 400× magnification. Immunopositive neurons and glial cells are demarcated by red arrow tips, and immunonegative cells are presented by blue arrow tips.
5. Discussion
The present findings indicated that iDMP suppressed the GR expression at the ipsilateral spinal cord following the PSL procedure and prolonged pain response time during hotplate stimulation. The immunostaining examination revealed the lower value of glucocorticoid receptor in the treated group compared to the sham-treated and vehicle-treated groups.
Nerve ligation is the most common method of modeling neuropathic pain; however, it is performed by different techniques regarding a specific purpose (29). In this regard, the PSL technique is particularly advantageous as it produces inflammation and neuropathic pain (27), as evidenced by thermal hyperalgesia (an over-reaction to weak heat stimuli) (28, 30, 31) without paralysis and spares most motor fibers (27, 32), thereby allowing for motor assessment by paw withdrawal. Both PSL groups developed progressive thermal hyperalgesia from days 1 to 7 before the treatment; however, iDMP increased the withdrawal onset time (reduced hyperalgesia) in the sham group, while the withdrawal time in the vehicle-treated group plateaued a lower level (~6 s). Accordingly, iDMP completely mitigated the PSL-induced hyperalgesia. Withdrawal time was also irreversibly decreased in the sham group, according to Campbell and Meyer (33), who explained this reaction as a memory-dependent response to repeated exposures rather than hyperalgesia. Alternatively, post-surgical pain may also play a role; however, this would not differ among the groups.
The thermal withdrawal response was measured at the same interval, following the saline/iDMP injection to control for variation in drug onset, with a 30-min break as the concerned interval in a previous study was the average time to have effects (34). Adecrease in withdrawal time (hyperalgesia) in a previous study was attributed to the lag in blocking NMDARs and other targets (35). While there was still a difference between the sham and treatment groups on day 8, the difference disappeared by day 8, suggesting that the suppression of hyperalgesia was caused by both the acute inhibition of NMDAR-mediated glutamatergic transmission and slower inhibitory effects on neuroplasticity (35). From the second treatment day (day 9 after PSL) onward, no difference was noticed regarding the thermal response in the sham group, indicating that 10 nmol DMP is sufficient to reverse central sensitization completely.
Dextromethorphan binds to NMDAR with low affinity due to its relatively fast unblocking kinetics (16, 17, 36). Dextromethorphan blocks the ion channel and acts non-competitively with glutamate, thereby blocking calcium influx and ensuing neuroplasticity underlying the development of neuropathic pain, even under enhanced glutamate release conditions (25). Rogawski (16) reported that low-affinity inhibitors could provide better protection against neurological damage with minimal behavioral effects in animals compared to higher-affinity NMDA receptor antagonists. The superior effects of the DMP profile stems from the use-dependence of the block, allowing relatively higher efficacy under high receptor activity and pathological depolarization conditions, compared to basal activity (16). Several studies have documented that iDMP has a greater therapeutic activity and a better safety profile than other inhibitors (16, 17). In a systematic review using NMDAR antagonists for pain management, Larsen et al. (37) reported a small favorable outcome. However, neuropathic pain requires higher doses, which increases the potentials for adverse effects. In addition to low affinity, iDMP is more lipophilic than other NMDA receptor antagonists; hence, it may reach the target site more rapidly after the intrathecal injection at L4 - L6 (35). Alternatively, the higher-affinity drugs such as Ketamine demonstrate slower channel unblocking activity, resulting in greater channel inhibition, even when ongoing activation is low. The NMDAR antagonist MK-801 blocks the NMDAR ion channel irreversibly due to its less flexible chemical structure than Ketamine (36), thus potently inhibiting ion flux and downstream signaling, including neuroplasticity-inducing signals (38). However, these agents have much stronger side effects, such as dramatic alterations in behaviors, perceptions, and cognition.
The spinal dorsal horn is the first relay for the nociceptive information from the periphery to the brain (15). Immunohistochemistry staining revealed a further four-fold increase in the expression, following the PSL and vehicle treatment concomitant with a 50% reduction in thermal withdrawal time, compared to the sham group. Furthermore, studies have reported a marked decrease in Mg2+ ion blockade and the prolonged opening of ion channels following neuropathic pain induction, resulting in the enhanced polarization of dorsal horn neurons and an increase in NMDARs. The increase in postsynaptic excitability may also enhance transmission from normal convergent pathways, resulting in secondary hyperalgesia (17, 39, 40).
Glucocorticoid receptors regulate the inflammatory response after injury and are widely expressed in the spinal cord, including the dorsal horn (26, 28). Further, GRs are upregulated in the spinal cord following the nervous system’s injury and relevant pain conditions (27). A radioligand study also showed that the GRs-DMP bonds indicate the association between the DMP administration and the GR expression suppression. Several studies have declared the activation of GRs in the posterior spinal cord during the development and maintenance of neuropathic pain aroused by peripheral neural lesions, and such upregulation plays a critical role in the onset of central sensitization under chronic pain conditions, thereby causing the increased transmission of pain fibers. The utilization of DMP as an NMDAR antagonist is to reduce the transmission, resulting in a lower pain threshold (41-44). To sum up, the GR expression was dramatically upregulated by PSL, a response abrogated almost completely by iDMP.
Similarly, the glucocorticoid receptor expression increased in the hippocampus after focal brain injury (23) and in the spinal cord after peripheral nerve injury (24). Moreover, glucocorticoid receptors can have neurotoxic effects (45). Peripheral GRs are also critical for inflammatory regulation by interactions with intracellular elements such as activated protein-1 (22). The precise contributions of the GR upregulation to neuropathic pain and the therapeutic relevance of DMP binding remain unclear and warrant further studies.
Dextromethorphan has several additional advantages as a neuropathic pain treatment, in addition to its use-dependent activity described above. First, it is easily accessible and has well-documented clinical efficacy and safety at therapeutic doses (26, 46). In this regard, Weinbroum et al. (47) reported the use of iDMP as a painkiller, albeit at doses above the required level for an antitussive effect. Dextromethorphan also has lower neurotoxicity than many other strong analgesics, and its safety and efficacy in the mice model are also confirmed (47).
As one of the limitations of the present study, a single dose was only used for the iDMP administration, and the possibility of varying doses and their effects were not addressed. The GR receptors were quantified using visual calculation on multiple high power field with 400× magnifications using a light microscope. We did not quantify the GR expression using another protein quantification means due to the limitation in our research laboratory and available facilities during the study. Further studies are recommended to use other protein quantification means to produce a more accurate and objective result at the GR expression level.
5.1. Conclusions
The intrathecal administration of dextromethorphan suppresses partial sciatic nerve ligation-induced hyperalgesia and concomitantly reduces the associated upregulation of glucocorticoid receptors in the spinal cord. Given its use-dependent pharmacokinetics and well-established safety profile, iDMP should be considered in the treatment of neuropathic pain.