1. Background
Optimization of perioperative fluids is critical for the prevention of adverse effects such as hypovolemia, pulmonary edema, and reduced tissue oxygenation in both adult and pediatric patients (1-3). The recent progress in the minimally invasive surgery has resulted in an evident reduction in the intraoperative evaporative fluid losses and requirements (4). This raised a considerable debate for perioperative intravenous (IV) fluid management whether to use a traditional or a more restrictive therapy (5). Caution must be taken in children during perioperative fluid management because of the unique characteristics of their physiology, including less myocardial and pulmonary reserve (6).
Although some studies have compared the restrictive and conventional fluid therapy in adult cases, few studies have compared the effects of these strategies in pediatric cases. Their results have suggested that perioperative conventional fluid therapy impaired pulmonary functions in pediatric patients. However, no ultrasound or other diagnostic modalities were used to evaluate the lung condition (7, 8).
2. Objectives
In this study, we used the lung ultrasound (LUS) to investigate the preference of the intraoperative and immediate postoperative volume impact for either the conventional or moderately restrictive fluid replacement regimens in pediatric patients who were subjected to a relatively long surgical procedure with limited volume loss (hypospadias repair).
3. Methods
3.1. Study Population
An institutional research ethics committee approval was acquired. Informed written consent was obtained from the guardians of all participants. The study was registered at clinicaltrials.gov with (ID: NCT04444089).
3.2. Design
This prospective, double-blinded, randomized controlled study was done in the theater of paediatric surgery at Kasr Elainy hospital, Faculty of Medicine, Cairo University, Egypt from August 2019 to February 2020.
The patients were assigned into two equal groups of CG (n = 40) and RG (n = 40) based on computer-generated randomization codes kept in sealed envelopes (EPIDAT 4.1). These envelopes were provided to the anesthetist in charge of preparing the fluid regimens by an investigator that was not involved in patient care.
3.3. Inclusion and Exclusion Criteria
This study included 80 pediatric patients with the age of 3 - 6 years, scheduled for repair of penile hypospadias, with the American Society of Anesthesiologists' (ASA) physical status class I (normal healthy patients) – II ( Patients with mild systemic disease).
Patients with mean baseline B-line numbers ≥ 3, a family history of allergy to local anesthetics, and pulmonary, cardiovascular, or hematological diseases were excluded from the study.
3.4. Preoperative Management
The parents were instructed to make their children fast from solid food for six hours and water for two hours before the surgery. In the operation room, hemodynamic variables were monitored for all study participants, including the measurements of blood pressure (BP), electrocardiography (ECG), and pulse oximetry. In all patients, a peripheral intravenous (IV) line was secured for general anesthesia (GA) and IV fluid administration.
3.5. Intraoperative Management
GA was induced using a combination of 2 mg/kg propofol, 0.2 mg/kg cisatracurium, and 1 μg/kg fentanyl. After intubation, ventilation was done mechanically with 6 mL/kg tidal volume. After securing the endotracheal tube, patients were positioned in the lateral position, and caudal analgesia was administered under the guide of sonography using 1 mL/kg of 0.25% plain bupivacaine, then repositioned in supine position. Anesthesia was maintained by sevoflurane 1.8% and cisatracurium 0.02 mg/kg, given at 30-minute intervals. Additional fentanyl 0.5 μg/kg up to 4 μg/kg was given incrementally if the heart rate (HR) or systolic blood pressure (SBP) was > 20% of the baseline in response to surgical stimulus.
A urinary catheter was inserted in all cases. BP, HR, and oxygen saturation (SpO2) were monitored non-invasively, and readings were documented at 10-minute intervals. Urine output was recorded every hour throughout the operation.
Fluid therapy was given according to the study group by the anesthesiologist who received the sealed envelopes. Another anesthesiologist, who was blinded to the study aims, recorded the data. The fluid regimens given in both groups are described in Table 1.
| Fluid Regimens | |
|---|---|
| Conventional Group (n = 40) | Restrictive Group (n = 40) |
| Received Ringer's lactate as the maintenance volume, at a rate of 4 mL/kg/hour for the first- 10 kilograms of patient’s weight, 2 mL/kg/hour for the second- 10 kilograms of body weight, and 1 mL/kg/hour for each further kilogram. The volume of the deficit was calculated as (the volume of maintenance × fasting hours). Fifty percent of the deficit was given in the 1st hour, 25% in the 2nd hour, and 25% of the deficit volume in the 3rd hour. | Received Ringer's lactate at a rate of 3 mL/kg/hour from the beginning to the end of the surgery (9). |
Fluid Challenge Strategies in the Two Groups a
3.6. Postoperative Management
Patients were transported to the recovery room. BP, HR, and SpO2 readings were documented every 5 minutes for the first postoperative 20 minutes. Recovery score was evaluated and recorded at 10 and 20 minutes postoperatively by an independent anesthesiologist, who was blinded to the used regimen. The Steward Simplified Post-Anesthetic Recovery Score (10) was evaluated by calculating the value of the following variables: (1) consciousness level (0: non-responder; 1: responder to stimuli; 2: awake); (2) airway (0: requires maintenance; 1: maintenance of an adequate airway; 2: coughing or crying); (3) and movement (0: no movement; 1: no purposeful movement; 2: moving limbs purposefully).
3.7. Lung Ultrasound (LUS)
LUS was performed with a curvilinear probe at two-time points. The first point represented the baseline reading, which was just after intubation and securing the airway, and the second point was at the end of the surgery. This was done by one of the authors, who is an expert in diagnostic ultrasound. The LUS operator was blinded to the patient’s group. LUS was done while the patient was lying supine. The probe was set on the midaxillary line on both sides of the chest wall at the level of the 6 - 7th intercostal space. The ultrasound probe frequency was 4 - 12 MHz with a model type (AcusonX300, Siemens Korea, Seoul, South Korea). The mean number of B-lines detected on the ultrasound was recorded.
The primary endpoint of the research was the appearance or absence of new B-lines on LUS. The secondary outcomes were the number of B-lines, Steward Simplified Post-Anesthetic Recovery Score, urine output, SpO2, BP, and HR.
3.8. Statistical Analysis
The findings of this study were presented using descriptive and inferential statistics. The group means for quantitative variables were evaluated by the unpaired student's t-test. The qualitative data were presented as numbers and percentages and evaluated using the chi-square and the Z tests. All statistical analyses were done by the SPPS software (IBM Corp., Armonk, NY, USA), version 1.0.0.1406. All statistical tests of inference undertaken were interpreted at a 0.05 statistical significance level. Since there were no similar articles assessing the primary outcome of the proportion of cases that had B-lines on LUS postoperatively, a pilot study was performed on 10 patients in each group; the results showed a 43% difference in the proportion of cases that had new postoperative B-lines. Accordingly, the sample size was calculated to be a minimum of 72 patients, which was raised to 80 patients to compensate for the dropouts, by Z test with a two-tailed alpha (0.05) and a power of 97% with the use of G power 3.1.9.4 software.
4. Results
The demographic data of patients (Table 2) showed no significant difference between the groups in age, weight, and duration of surgery. After performing LUS, B-lines were observed in 29 (72.5%) patients in the CG and 11 (27.5%) patients in the RG (P < 0.001). The mean number of B-lines was significantly higher in the CG (P < 0.001; Table 3). There was a positive correlation between the infused volume and the count of B-lines (R = 0.4747 and R2 = 0.2253).
| Variables | Conventional Group (n = 40) | Restrictive Group (n = 40) | P-Value |
|---|---|---|---|
| Age (y) | 3.9 ± 0.8 (95% CI: 3.65 - 4.15) | 4.1 ± 0.9 (95% CI: 3.8 - 4.4) | 0.09 |
| Weight (kg) | 14.9 ± 3.5 (95% CI: 13.8 - 26) | 15.7 ± 3 (95% CI: 14.8 - 16.6) | 0.06 |
| Total infuse volume (mL) | 353.4 ± 66.2 (95% CI: 332.9 - 373.9) | 131 ± 29.9 (95% CI: 121.75 - 140.25) | < 0.0001 |
| ASA, No. (%) | 0.81 | ||
| I | 28 (70) | 26 (65) | |
| II | 12 (30) | 14 (35) | |
| Duration of surgery (min) | 120.8 ± 31.5 (95% CI: 111 - 130.6) | 116.8 ± 22.6 (95% CI: 109.4 - 121.8) | 0.60 |
Patients’ Demographic Data
| Variables | Conventional Group (n = 40) | Restrictive Group (n = 40) | P-Value |
|---|---|---|---|
| Rate of occurrence of positive ultrasound lung findings, No. (%) | 29 (72.5) | 11 (27.5) | < 0.001 |
| B-line count | |||
| Baseline | 0.6 ± 0.9 (95% CI: 0.3 - 0.8) | 0.4 ± 0.7 (95% CI: 0.2 - 0.6) | 0.5 |
| At the end of surgery | 3.1 ± 2.2 (95% CI: 2.4 - 3.8) | 1.3 ± 2.2 (95% CI: 0.6 - 2) | < 0.001 |
| Recovery score | |||
| At 10 min | 4 ± 0.9 (95% CI: 3.7 - 4.3) | 4.3 ± 0.7 (95% CI: 4 - 4.6) | 0.05 |
| At 20 min | 4.9 ± 0.8 (4.7 - 5.2) | 5.8 ± 0.4 (95% CI: 5.7 - 5.9) | < 0.001 |
| Urine output (mL/kg/h) | 3.2 ± 0.8 (95% CI: 2.9 - 3.5) | 3 ± 0.7 (95% CI: 2.8 - 3.2) | 0.07 |
| Hospital stay (h) | 31.3 ± 9.8 (95% CI: 28.3 - 34.3) | 33.3 ± 10.9 (95% CI: 29.9 - 36.7) | 0.07 |
The Numbers and Rate of Occurrence of Positive Ultrasound Lung, Urine Output, Recovery Scores, and Length of Stay in the Two Groups
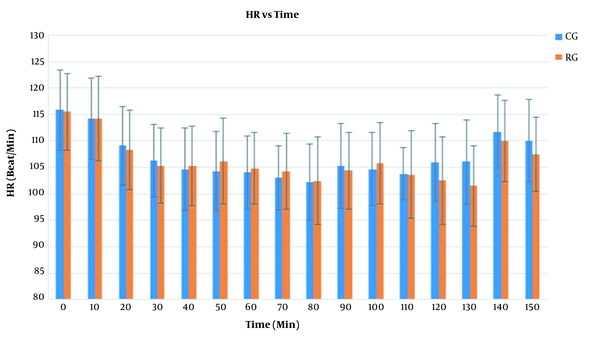
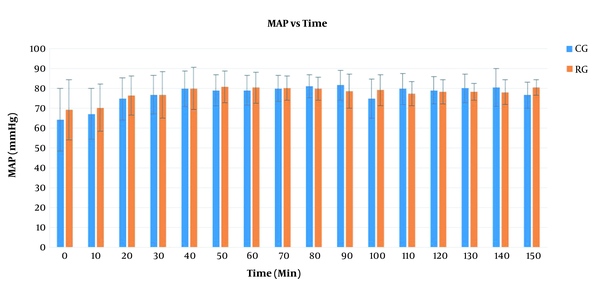
Furthermore, there was an insignificant difference between the groups regarding HR with F(1) = 0.06 and P = 0.8 (Figure 1). The mean arterial pressure (MAP) had an insignificant difference between the groups with F(1) = 0.2 and P = 0.6 (Figure 2). There was no significant difference between the groups regarding the length of hospital stay. Urine output was higher in the CG group compared to the RG group, though it was not statistically significant (Table 3). The SpO2 values were insignificant between the two groups with F(1) = 0.7 and P = 0.8. However, 12 patients in the CG required an oxygen mask for 30 minutes postoperatively to maintain the saturation within normal limits. The recovery score was comparable in both groups at 10 minutes. However, the RG group had a significantly higher recovery score at 20 minutes (Table 3).
5. Discussion
This double-blinded, randomized controlled trial was done to explore the preference of the intraoperative conventional and restrictive fluid replacement regimens. Using LUS, the comparison was done to detect the volume impact in both regimens. The chosen study population had challenging characteristics, comprising pediatric patients undergoing a procedure with relatively long duration and limited volume loss, such as hypospadias repair.
In this study, the hypothesis was that a moderate restriction of IV fluids would have less lung congestion and a better recovery score with no deleterious effect on hemodynamics or urine output. This hypothesis was supported by the results that showed normal lung morphology in the RG with fewer B-lines and lower percentage of patients showing B-lines in RG than those in the CG. The recovery score was higher in the RG after 20 minutes during the postoperative period. Arterial blood pressure (ABP), HR, and urine output showed no statistically significant difference between the groups.
Fluid resuscitation in children shows a different myocardial response from adults. Their cardiac muscle is immature due to a lesser contractile to non-contractile ratio, prevalent circular-shaped muscles, decreased myocardial compliance, and less calcium flow regulation. Thus, during volume loading, the immature cardiac muscle has a relatively weaker support to cardiac output (11).
The effect of hypervolemia is most noticeable in the lungs, where pulmonary congestion induces increased workload, decreased compliance of the lungs, and deterioration of the oxygenation index (12, 13).
Children are sensitive to volume depletion due to the immaturity of hypothalamic osmoreceptors and juxtaglomerular apparatus, in addition to the higher proportion of water in their body weight (6).
As perioperative fluid replacement is still a focus of much debate, especially among children (5), intravenous fluid therapy should be strictly monitored and adjusted as necessary to maintain hemodynamic stability. The major parameters monitored include ABP, Spo2, HR, capillary refill time, and urine output. However, these parameters are influenced by other factors rather than fluid therapy (14).
There is a large data supporting tailored goal-directed fluid therapy to optimize volume status and tissue perfusion. However, to be accurate, this needs advanced monitoring with adequate training and may require invasive procedures (15, 16).
LUS is a non-invasive diagnostic modality, which can reduce the perioperative complication rate (17). It is able to assess the condition of the lungs and its associated vasculature in a simple way. The appearance of B-lines provides a quantitative and qualitative estimation of the status of the lungs and the cardiovascular system (18).
Although several studies have compared restrictive and liberal fluid therapy in adult patients, few studies have compared the effects of these strategies in pediatric patients (19, 20).
Li et al. showed that liberal fluid resuscitation increased the incidence of mortality and in-hospital stay at a 4-week follow-up in pediatric patients, but the cause of such mortality remained unexplored (7). Likewise, relative to the liberal regimen, the restrictive fluid regimen was associated with less spent time on the ventilator and less time in the pediatric intensive care unit (ICU) as evidenced by Sankar et al. and the Fluids and Catheters Treatment Trial (FACTT) (8, 20).
The use of a restricted fluid technique resulted in a rise in the number of pulmonary procurements without detrimental effects on the function of kidney transplants in an observational study of brain-dead organ donors (21). Fluid overload was positively associated with oxygenation index deterioration at 15% relative volume accumulation in a research done on 80 mechanically ventilated pediatric patients (12). Doherty et al. proved that postoperative complications were associated with greater cumulative positive fluid balance (22). However, no ultrasound or other diagnostic modalities were used to investigate the associated lung pathology.
The main limitations of our study were the investigation of only patients undergoing a single type of surgery, targeting a specific age group, and inability to perform a long-term patient follow-up. Sweating, stress, and dehydration as confounding variables were not excluded. Goal-directed therapy was out of focus in this study. The sample size calculation was based on the data of a pilot study; however, based on the final results of this study, a lagrger sample size may be needed for a better clarification of our findings. Thus, further studies are needed to determine the appropriate individualized hydration regimens using ultrasound utilities in different types of pediatric surgery.
5.1. Conclusion
Although the ABP, urine output, and HR did not differ between the two groups, the number of B-lines on LUS was significantly lower, and the recovery score at 20 minutes was higher in the restrictive group. This might suggest that a moderate restrictive fluid regimen can reduce the risk of pulmonary dysfunction and improve the recovery scores in the patient population targeted by the study.