1. Context
Aspirin (ASA) is one of the first drugs that has come into a routine therapeutic protocol treatment since the willow bark was used as an agent by the ancient Sumerians and Egyptians in folk medicine (1). Due to its low toxicity and inexpensive cost, ASA is mainly touted as a widely used drug worldwide (2). ASA was a principal pharmaceutical achievement in the 20th century and has been prescribed since then for various purposes, particularly for inhibiting cardiovascular and cerebrovascular pathologies (3). However, the prospective role of ASA in decreasing the long-standing risk of numerous cancers has plausibly opened up a novel era (4). Also, mounting evidence has been developed about the importance of ASA consumption in women at high risk of preterm preeclampsia (5). Although the conventional prescription of ASA is due to its analgesic, antipyretic, and anti-inflammatory effects, some neurodegenerative disorders, such as Alzheimer’s disease, are also considered other pathological conditions for ASA medication (6). Herein, we tended to highlight all clinical applications of ASA in dealing with anesthesiologists.
2. Mode of Action
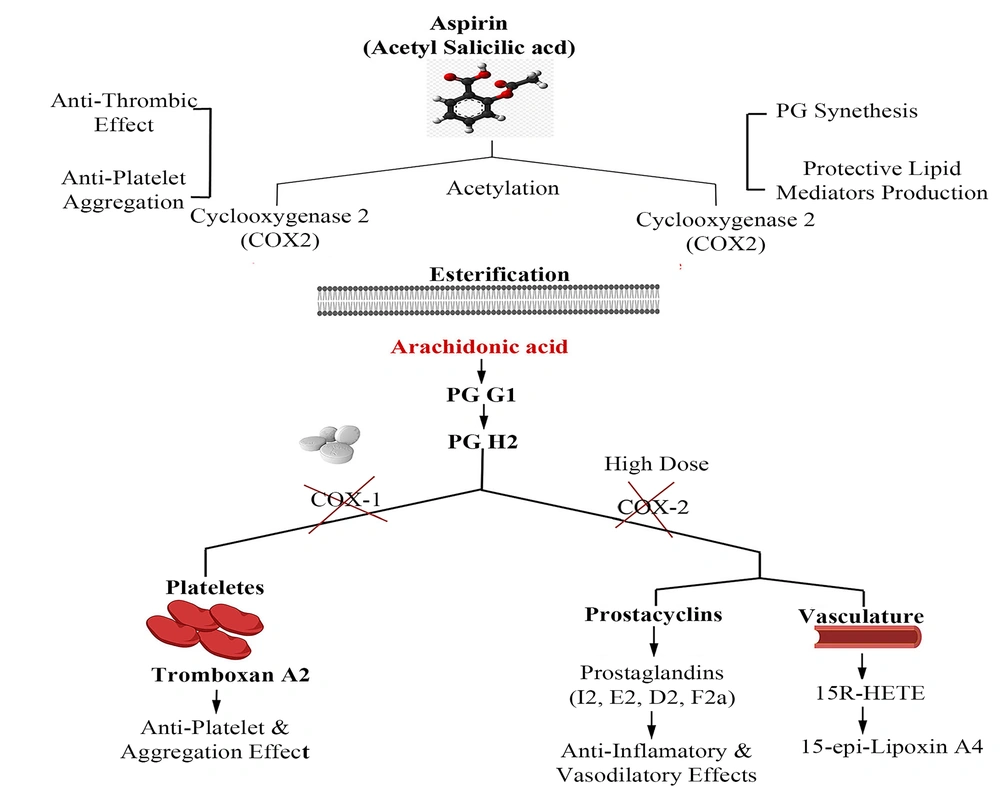
Under pathophysiological conditions, arachidonic acid (AA), as a prostaglandin (PG) precursor, is released from the cell membrane by the activity of an enzyme named phospholipase A2 (7). Various PGs, e.g., PGG2 and PGH2, are synthesized by the cyclooxygenase isoenzymes (COX-1 and -2) involved in inflammatory responses. The essential activity of COX-1 and -2 is diminished in the presence of ASA after the irreversible acetylation of a serine residue in the active site of AA-binding channels (8).
Since platelets have no DNA content, they cannot synthesize new proteins; thus, the inhibition of COX-1 by ASA is associated with the whole platelet lifetime (~ 7 - 10 days) (9). To be specific, ASA can quench the COX-2 activity directly by acetylating the serine residue (Ser 516), which can, in turn, promote 15 (R)-HETE synthesis (10). It should also be noted that the low-dose administration of ASA is partially effective on COX2/PGI2-mediated vascular modulation. In Figure 1, the mechanism of action of ASA is elucidated in detail.
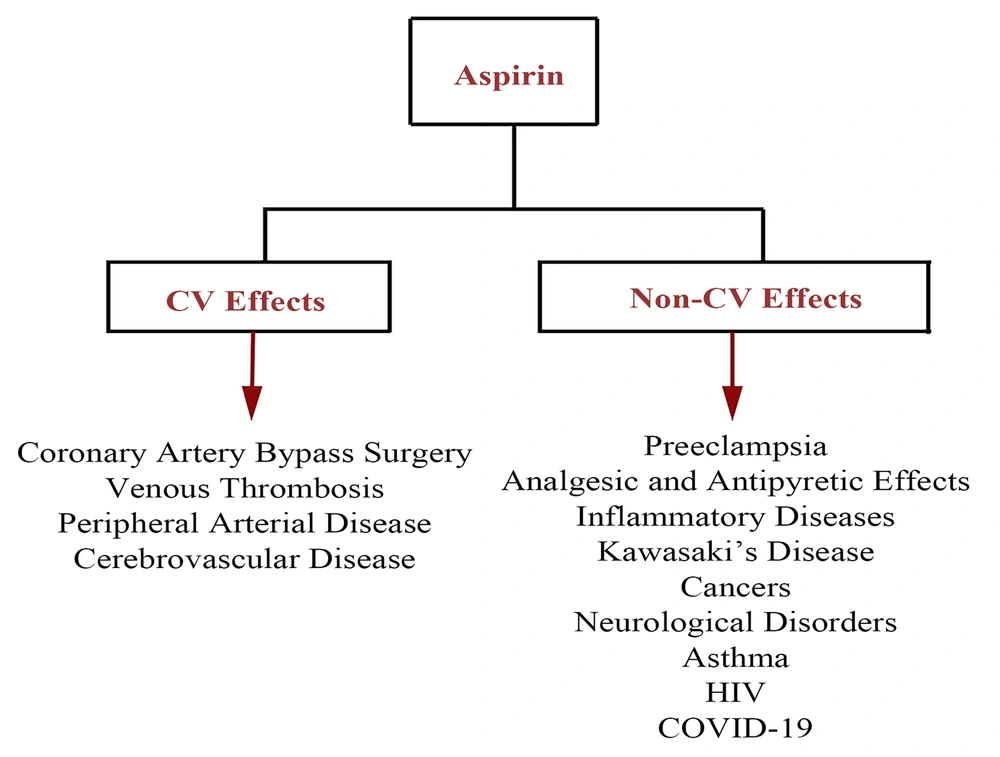
3. Cardiovascular Related Beneficial Effects
ASA is well known to decrease cardiovascular events (11), exerting multiple beneficial effects in different dose ranges. For example, it can prevent COX-2 more effectively than COX-1 at high doses (> 1000 mg/day) (12), while low-dose ASA (75 - 100 mg/day) inhibits COX-1 (13). The role of ASA in reducing cardiovascular disease (CVD)-related mortalities and acute myocardial infarction recurrence was shown first in an ISIS-2 clinical trial (14). However, the role of ASA in primary CVD prevention is challenging because of conflicting effects on CVD mortality rates (15). Mounting evidence suggests that the apparent benefits of ASA as a therapy modality for primary CVD prevention outweigh its less significant disadvantages causing the risk of some life-threatening conditions such as bleeding (16-19). A meta-analysis of 20 clinical cases including 3000 patients who received ASA as secondary cardiovascular prevention indicated that “aspirin resistance” in 28% of the patients correlated with a four-fold risk of CVD (20, 21). ASA can be prescribed in numerous cardiovascular issues, including coronary artery bypass surgery (22-26), venous thrombosis, peripheral arterial disease, and cerebrovascular diseases (27-29).
4. Extra-Cardiovascular Applications
4.1. Preeclampsia
Preeclampsia (PE), one of the leading causes of maternal and fetal mortality, is a significant risk factor for preterm birth (30). PE is identified by hypertension, proteinuria, and an extreme maternal systemic inflammatory response (31). ASA is recommended as an effective therapeutic candidate due to its anticoagulant and anti-inflammatory potentials (32). It was also assumed that ASA (50 - 150 mg) could improve the placental blood flow and reduce the risk of placental thrombosis through decreasing TXA2 and PGI2 contents (33). ASA-triggered lipoxins (ATLs) play a critical role in reproductive medicine. In this regard, several studies have revealed that exogenous ATL has the benefits of reversing inflammatory responses observed in PE (34). Also, 150 mg ASA remarkably reduces the prevalence of preterm prelabor rupture of membranes in women at high risk of early-onset PE (35). ASA should be used before week 20 and over at week 34 of pregnancy without any substantial complication to prevent the high risk of perinatal bleeding following its administration (36). The American College of Obstetrics and Gynecology and the Society for Maternal-Fetal Medicine recommended that low-dose ASA (81 mg/day) should be initiated between 12 - 28 weeks of gestation and continued daily for PE prevention until the delivery time and prophylaxis induction in women with a high risk of PE (37). In the ASA for Evidence-Based Preeclampsia Prevention (ASPRE) trial, treatment with ASA (150 mg/day) decreased the incidence of PE in 32, 34, and 37 weeks of gestation by approximately 90, 80, and 60%, respectively, while it had no significant effects on the incidence of term PE (38). According to the results of Combined Multi-Marker Screening and Randomized Patient Treatment with the ASPRE trial, ASA inhibits both preterm and term PE. Also, it can postpone the gestational age by 4.4 weeks (39).
4.2. Analgesic and Antipyretic Effects
ASA is successfully used as a painkiller for mild pains such as headache (40, 41), toothache, and menstrual cramps (dysmenorrhea). Also, it can be used to alleviate colds and "flu-like" symptoms due to its antipyretic property (42). However, ASA has been contraindicated as an antipyretic analgesic in children suffering from flu-like symptoms because of the observed higher risk of Reye’s syndrome (43). The anti-nociception property of ASA mainly exerts through divergent pathways, including the stimulation of adenosine A2 receptors; the suppression of the inhibitor of NF-κB (IkB) kinase β; and the translocation of NF-κB to the nucleus, acid-sensing ion channels (with high doses of ASA), and α, β-methylene ATP-induced nociception. The acetylation of COX-2 causes the production of ASA-triggered lipoxins (15-epi-lipoxin A4) and activity of the ASA-triggered docosahexaenoic acid pathway (e.g., neuroprotection D1/protectin) (44-46). It has also been revealed that ASA contributes to the analgesic effect through the salicylate-induced stimulation of adenosine monophosphate-activated protein kinase (47).
4.3. Inflammatory Diseases
4.3.1. Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disorder described by chronic synovitis, leading to tissue dysfunction and localized damage into the articular cartilage, bone, tendon, and ligament (48). Given that PGs are the primary mediators of pain and inflammatory responses in RA, ASA has been approved for the first-line treatment of RA (49). This drug can reduce pain in the early stage of RA via anti-inflammatory mechanisms without losing articular function (50). Besides inhibiting COXs, ASA can inhibit the synthesis of chondroitin sulfate, an essential component involved in cartilage formation (21). The standard therapeutic regimen for RA contains ASA (3 g) + ibuprofen (3200 mg) daily, which are taken separately (51). Furthermore, ASA affects cell proliferation and apoptosis by reducing cell viability and increasing the proportion of apoptotic and arrested cells in the G0/G1 phase in a dose-dependent manner (52). A recent study has reported that ASA substantially prevents the proliferation of RA-fibroblast‑like synoviocytes via inhibiting JAK/STAT3 and the NF-κB axis (52).
4.3.2. Osteoarthritis
Osteoarthritis is a chronic degeneration syndrome of synovial joints, influencing the cartilage, ligaments, joint lining, and surrounding bones (53-57). Although ASA can positively synergize the analgesic and anti-inflammatory effects of other NSAIDs, following a single-dose consumption of ASA (1g) along with typical OTC drugs, 6.3% of patients display various side effects (58). Moreover, there is a possible contraindication between the anti-thrombotic effect of ASA and the concomitant prescription of other NSAIDs (59), which can be clinically worsened when ASA is required in patients with increased risk of cardiovascular events (60). Also, several studies have revealed that low-dose ASA (< 100 μg/mL) is suitable for maintaining the bone mineral density through activating osteoblastic bone formation and preventing osteoclast activities in a COX-independent manner (61). However, the role of high-dose ASA (150 - 300 μg/mL) is challenging in bone self-regeneration due to its dual effects on osteoclast activities (61).
4.3.3. Systemic Inflammatory Response Syndrome
Systemic inflammatory response syndrome (SIRS) is caused by inappropriate immune responses, either infectious or non-infectious conditions, with occasionally an intense shock and multiple organ failure (33). The clinical manifestations of SIRS include sepsis, multiple organ failure, and acute respiratory distress syndrome (ARDS), which can benefit from early ASA treatment (62, 63). At the antiplatelet dose (81 mg daily), ASA can primarily decrease COX-1-mediated reactions, sepsis, and SIRS-related thrombotic risk (64). The prehospital use of ASA at low-to-medium doses (81 - 325 mg/day) is associated with a low rate of ARDS occurrence (65). On the other hand, ASA (100 mg/day) administration in patients admitted at the intensive care unit (ICU) leads to a significantly lower hospital mortality rate (64). Moreover, it has been reported that ASA at 75 or 1200 mg for seven days before the lipopolysaccharide inhalation decreases the pulmonary neutrophilia and matrix metalloproteinase-8/-9, the bronchoalveolar lavage, the concentrations of tumor necrosis factor-α, and systemic and pulmonary TXB2 (66).
4.4. Kawasaki’s Disease
Kawasaki's disease (KD) is an acute childhood vasculitis diagnosed with high fever, cervical lymphadenopathy, rashes, oral enanthema, conjunctivitis, and erythematous (67). Coronary artery (CA) dilatation and aneurysms may progress in ∼ 15 to 25% of untreated children to promote the upcoming thrombosis and myocardial infarction (68). ASA has been used to treat KD before the intravenous immunoglobulin (IVIG) treatment (69). In the acute phase of the disease, ASA is administered at anti-inflammatory doses (80 to 100 mg/kg per day) with IVIG (70). The anti-inflammatory effect of high-dose ASA can decrease the duration of fever and reduce hemoglobin levels (71). Low-dose ASA due to have a potent anti platelete effect should be continued until 6-8 weeks after the disease onset (72). Despite administrating high-dose ASA for the beginning of treatment, it is not usual to determine Reye’s syndrome in children with KD (73). A meta-analysis on ASA efficacy in KD showed that 20-40% of children developed CA aneurysms after monotherapy with ASA. However, treatment with ASA and high-dose IVIG (a single infusion at 2g/kg) decreases the incidence of CA aneurysms by 9 and 4% in 30 days and 60 days after the disease onset, respectively (74). Nevertheless, there is no randomized controlled trial regarding the use of high-dose ASA in the acute stage of KD.
4.5. Cancers
Previous studies supported the hypothesis that long-term ASA administration may prevent mortality caused by cancers (75, 76). A growing body of evidence has also shown that ASA is a promising chemo-preventive agent for treating numerous cancers, including colorectal, breast, lung, stomach, ovarian, hepatocellular, and prostate cancers (77). The underlying mechanisms responsible for the potential anti-neoplastic action of ASA are not entirely understood. Some possible mechanisms include the antiplatelet effect, the regulation of PGs, the phosphoinositide 3-kinase signaling pathway, and anti-inflammatory effects (78). It has been proposed that the anti-cancer impact of low-dose ASA is pertinent to the inhibition of preliminary neoplastic transformation and the anti-metastatic action, which are directly associated with the antiplatelet effect of low-dose ASA (79). Low-dose ASA instigates the inhibition of platelet activation through its capacity to irreversibly inactivate the COX-1 enzyme and signaling pathways involved in the aberrant expression of epithelial COX-2 in neoplastic lesions (80, 81). It is worth noting that ASA also prevents platelet aggregation by targeting COX-1, which may be necessary for the multi-step process of metastasis (82). Since platelet aggregations promote dynamic tumor cells in the vascular environment, the inhibition of platelet activity can presumably restrict tumor metastasis (83). However, Haykal et al. found that the administration of ASA for the primary prevention of cancer provided a higher risk of bleeding and did not decrease cancer-related mortality (84).
4.6. Neurological Disorders
Multiple progressive neurological disorders, including Alzheimer's disease (AD) and Parkinson's disease (PD), are mainly described by the accumulation of insoluble and misfolded proteins (85). Recently, it has been declared that ASA may mitigate both phosphorylation and aggregation processes through acetylating target proteins (amyloid-beta and tau proteins) and can reduce the risk of AD and PD occurrence (86). A recent study has indicated that ASA decreases superoxide dismutase (SOD-1) aggregation by acetylation in amyotrophic lateral sclerosis (ALS), as a fatal neurodegenerative disease (87). Moreover, it has been shown that ASA can postpone the appearance of the ALS symptoms, including reflex, coordination, and muscle strength deficits, in the experimental model of ALS (88).
4.7. Asthma
Aspirin-induced asthma (AIA) or aspirin-exacerbated respiratory disease (AERD) is mainly characterized by nasal polyposis, eosinophilic rhinosinusitis, hypersensitivity reaction, and asthma (89). The epidemiological reports have declared that up to 20% of asthmatic patients show hypersensitivity to ASA (89). Moreover, several desensitization protocols have been developed to treat AIA, particularly in patients who cannot avoid ASA consumption (90). ASA desensitization upon daily use is a desirable therapeutic option in most patients with AIA, especially in those with recurrent nasal polyposis (91). According to some novel protocols, the initial doses of ASA are considered between 20 - 40 mg or in combination with the intranasal lysine or ketorolac spray. The dose of ASA increases gradually every 1 to 3 h. Once a positive result is achieved, the primary purpose is to repeat the dose and raise it to 325 mg (92). Although 81 mg/day is an optimal dose to sustain ASA tolerance orally, patients with AERD need doses between 325 and 650 mg twice daily to achieve therapeutic goals for ASA desensitization (93).
4.8. HIV
Recent studies have shown the rising incidence of CVD-related morbidity and mortality in HIV-positive patients (94). Interestingly, low-dose ASA (81mg/day) for seven days promotes the reduction of platelet hyperactivity in patients subjected to the antiretroviral treatment and significantly decreases the number of CD4 positive lymphocytes and activated CD14 positive monocytes (95). However, a follow-up study of an ASA regimen for 12 weeks failed to show a therapeutic effect on inflammation biomarkers, monocyte activation, and the function of endothelial cells (96).
4.9. Coronavirus Disease 2019 (COVID-19)
Coronavirus disease 2019 (COVID-19), as a pandemic leading to a public health concern, is caused by severe acute respiratory syndrome coronavirus 2 (97-101). Regarding thromboembolic events following the cytokine storm, it was suggested that antiplatelet agents, such as ASA, could help prevent arterial thromboembolic complications (102-105). Following the previous comprehensive clinical evaluations, it has been revealed that the administration of ASA does not show significant efficacy in COVID-19 patients and may increase the severe bleeding risk in thrombocytopenic COVID-19 patients. In contrast, the recent literature has demonstrated that ASA can be a breakthrough drug to prevent coagulopathy events, particularly in high-risk patients. In this regard, the ongoing randomized evaluation of COVID-19 therapy (RECOVERY) trial in 176 medical centers in the UK has evaluated the possible therapeutic impact of ASA on hospitalized patients at risk of blood clotting complications (106) and assessed whether these benefits outweigh the potential side effects such as the risk of bleeding. In this line, the results of another clinical trial revealed that 44 and 43% of patients receiving low-dose ASA within 24 h of their admission and seven days before hospitalization were less likely to be under the mechanical ventilator and requiring for ICU admission, respectively (107). Similarly, a more recent cohort study has revealed that ASA use might be associated with improved outcomes, including decreased mechanical ventilation, the decreased need for ICU admission, and decreased in-hospital mortality in COVID-19 patients receiving ASA within 24 h of admission or seven days before admission (107). A schematic presentation of the CV and non-CV effects of ASA applications is shown in Figure 2.
4.9.1. Bullet Points in the Field of General and Neuraxial Anesthesia
It is a critical issue to argue preoperative ASA use in both elective and noncardiac surgeries. Given that the cessation of long-term ASA treatment results in adverse cardiac complications in patients at risk, it is necessary to explore factors associated with the decision to stop ASA ingestion (108). In this regard, it has been found that the administration of ASA before operations and during the early postoperative period has no profound effect on the mortality rate or nonfatal myocardial infarction but enhances the risk of severe bleeding (109). Kim et al. attempted to determine the optimal preoperative cessation time of ASA intake. They showed that platelet function could recover when ASA intake was stopped more than seven days before the operation. However, a preoperative platelet function test using the platelet function analyzer is not essential for these patients. Platelet function assay (PFA) testshould be performed in patients who discontinue ASA < 7 days before general anesthesia (110).
According to the recent guidelines regarding ASA safety in subjects undergoing neuraxial anesthesia and analgesia, it has been well-established that a wide variety of ASA doses are safe and do not show contraindication to neuraxial blocks in these patients. Also, the reported spinal hematoma depends on some underlying and predisposing conditions, e.g., a traumatic vascular injury. However, the risk of spinal hematoma can increase following a concurrent administration of multiple anticoagulation agents and antihaemostatic drugs. Also, the recommended safety time of these drugs should be monitored (111). Hence, a proper individualized assessment for ASA administration should be considered before interventions, including neuraxial analgesia or catheter removal, in patients receiving ASA. Moreover, the risk of ASA-induced asthma, as an emergency condition, during anesthesia is regarded as a big challenge for clinicians, highlighting the need for collaboration between anesthesiologists and allergists (112).
5. Conclusion
As the most commonly prescribed medication, ASA has a substantial role in preventing first and second recurrent CV events. Surprisingly, the extra-CV therapeutic effects of ASA were frequently evidenced during numerous clinical trials while treating divergent pathological conditions, such as cancers, inflammatory diseases, neurodegenerative disorders, asthma, and HIV, and managing global pandemic COVID-19 complications. During anesthesia, ASA use is not considered a preoperative concerning challenge; however, the individualized assessment of the cessation of long-term ASA should be a priority.