1. Background
Debilitating pain after mastectomy, due to cosmetic reasons, breast cancer, or physical correction in transgender patients, is mainly known as neuropathic pain syndrome and may continue for years after surgery (1, 2). The most common cause of pain after mastectomy is related to intercostobrachial nerve injury (3). This nerve is the lateral branch of the second intercostal nerve that innervates the axillary cavity and the inner arm area and has been reported to be injured in 80% to 100% of patients undergoing axillary dissection due to mastectomy (3, 4). Because of the anatomical path of this nerve, pain after mastectomy is felt in the axillary area and the upper inner part of the arm, and can last three (5) to six months or more (6). In addition to the damage to the intercostobrachial nerve, involvement (trapping) and pressure on this nerve, due to scarring after mastectomy (7) and hematoma in the axillary area (8), can cause pain. The mental condition of patients who undergo a mastectomy due to breast cancer may also cause and exacerbate pain sensation (9).
Such pain can cause atelectasis, nausea, vomiting, restlessness (10) and if persistent, mood disorders, disruption of daily work, reduced physical activity, changes in quality of life, and chronic pain or Post-mastectomy Pain Syndrome (PMPS) (9, 11, 12); therefore, its treatment is essential (13-16). Post-mastectomy pain treatment includes a range of measures such as physical, psychological, and pharmacological therapies (17-19), as well as blocking the nerves involved in innervating the breast tissue and surrounding structures (20, 21) and the use of adjuvant drugs for the prolongation of the duration of pain relief and the decrease of toxicity of high doses of local anesthetics (22, 23). The most important such nerves are the intercostobrachial nerve (24) and the pectoral nerve (25). Blocking the intercostal nerve, which innervates the breast and adjacent tissues in the chest wall, is an effective technique for controlling pain after mastectomy (24). The serratus anterior block has been shown to reduce chest wall pain after mastectomy (26, 27). Despite its effectiveness in pain control, there is a risk of serious complications with this technique, such as systemic toxicity, pneumothorax, and hematoma or bleeding (28, 29). A pectoral nerve block is the best alternative to intercostal nerve block (ICNB) (30, 31) because it does not have serious side effects related to ICNB despite the same effectiveness (32, 33). The pectoral nerve block is performed at two anatomical sites, including (a) between the pectoralis major and minor muscles (called Pecs I block) and (b) between the pectoralis minor and serratus anterior muscles (called Pecs II block), which effectively blocks the entire region of the breast and all related nerve branches such as the internal and external pectoral nerves, the long thoracic nerve, the intercostal nerves from T2 to T6, and the thoracodorsal nerve (34). The nerve can be blocked both traditionally, based on anatomical pathways and physician experience, and with an ultrasound guide (35). However, the pectoral nerve block is mainly performed with an ultrasound guide because the use of ultrasound would not only facilitate the tracking of relevant nerve pathways but also effectively reduce the possibility of complications during the procedure (36, 37).
Transgender is a term used to describe people who do not fit within the confinements' characteristics. These people are subjected to various discriminations and have to deal with greater numbers of physical and psychological problems compared to others. Therefore, they undergo medical and surgical treatment. It must be noted that due to psychological challenges, stress storming from components of their environment can increase the intensity of pain perception in such patients. Thus, controlling and decreasing pain is of great importance to these patients (38).
2. Objectives
Due to the effectiveness and high accuracy of pectoral nerve block using ultrasound guidance for pain control after mastectomy and its safety compared to ICNB, we conducted this study to evaluate the effect of the two methods on pain score, the total analgesic requirement in the first 24 hours after surgery, the duration of hospital stay, and the incidence of nausea and vomiting in the first 24 hours after surgery.
3. Methods
3.1. Study Design, Patients, and Ethics
This was a single-blind randomized clinical trial. In total, the study enrolled 50 patients referred to the Breast Surgery Clinic of Firoozgar hospital who were candidates for transgender mastectomy between May 2020 and February 2021. Study approval was done by the Ethics Committee of the Iran University of Medical Sciences (Code: IR.IUMS.FMD.REC.1399.119). It was also registered in the Iranian clinical trial registration system (IRCT code IRCT20151107024909N10). Written informed consent was obtained from all patients after a detailed explanation of the study to the patients. The members of the research team participating in this study adhered to the ethical principles of the Declaration of Helsinki during all stages.
According to the study of Wijayasinghe (39), the average doses of meperidine used in two groups with and without pectoralis block were 40.4 ± 0.69 mg and 5.00 ± 0.98 mg, respectively. Considering the alpha cut-off of 0.05 and a power of 80%, the sample size was calculated to be 23 for each group, but because of the possibility of sample dropout, we mustered 50.
3.2. Study Outcomes
The primary outcomes of our study were the effects of the two methods on pain score and the total analgesic requirement in the first 24 hours after surgery, and the secondary outcomes were the duration of hospital stay and the incidence of nausea and vomiting in the first 24 hours after surgery.
3.3. Eligibility Criteria
The inclusion criteria included age between 18 and 50 years, transgender confirmation, being single, the need for mastectomy, and ASA I-II. The exclusion criteria included age under 18 years or over 50 years, obesity (BMI above 35), history of allergy to anesthetics used or any contraindications to nerve block (including a history of hemorrhagic disease, kyphoscoliosis, or herpes zoster), psychiatric disorders, use of narcotics and sedatives, smoking, cancer, chronic pain syndrome, previous breast surgery, and infection at the injection site.
3.4. Patient Grouping
The patients were randomly divided into two groups based on a random table of numbers:
(A) Ultrasound-guided type-II pectoral nerve block (Ultrasound-guided Pecs II block): Patients in this group were subjected to ultrasound-guided Pecs II block with an injection solution containing 20 ml of 0.2% ropivacaine (Multeni, Italy) on each side.
(B) Intercostal Nerve Block (ICNB): Patients in this group were subjected to ICNB with 9 ml of injection solution containing 0.2% ropivacaine (Multeni, Italy). Blocking was performed in three intercostal spaces T3, T4, and T5 (3 ml of ropivacaine 0.2% in each space) in a traditional way based on anatomical hallmarks and surgeon's experience (without ultrasound guidance) on each side.
3.5. Preparing Patients Before Performing ICNB or Ultrasound-guided Pecs II Block
All patients were induced with midazolam 30 µg/kg, fentanyl 2 µg/kg (as a premedication), propofol 2 mg/kg, and atracurium 0.5 mg/kg. Intubation and anesthesia were performed by the infusion of propofol 100 µg/kg/min and atracurium infusion 0.5 µg/kg/min. In this study, patients received 50 µg of fentanyl every hour until nerve block, and they did not receive any other opioids. During the operation, the bispectral index (BIS) was constantly checked to be maintained in the range of 40 to 60.
3.6. Ultrasound-guided Pecs II Block
At the end of the operation, in the supine position, the patient's hand was placed at a distance of 90 degrees from the body. Using a 6 - 13 MHZ linear probe of the ultrasound device (Micromax, Sonosite, USA), which was placed diagonally on the infraclavicular region (upper of the midclavicular line), the muscles were determined from the surface to the depth, pectoralis major, pectoralis minor, axillary artery, axillary vein, and pleura, in sequence. With the in-plane technique, a 22-gauge needle (Echogenic nerve block needle, SPECTRA Medical Devices, USA) Echogenic nerve block needle, SPECTRA. The syringe was inserted between the two muscles, and 10 mL of ropivacaine 0.2% was injected to block the internal pectoral nerve (C8 and T1), as well as the external pectoral nerve (C5, C6, and C7). The probe was moved infero-laterally until the serratus anterior muscle appeared at the level of the third rib and in a greater depth than the pectoralis minor muscle, and 10 ml of ropivacaine 0.2% was injected between the two muscles on both sides.
3.7. Intercostal Nerve Block
After reconstructing the breast tissue on both sides and before suturing the skin, the surgeon injected 3 mL of 0.2% ropivacaine at the same time in the T3 to T5 intercostal spaces (both sides). After the blocking procedure, skin sutures were performed by assistants simultaneously on both sides. In two groups with the onset of suturing, the atracurium infusion was discontinued, and all patients were routinely treated with a gradual reduction in propofol infusion dosage. After that, the muscle relaxant was reversed, the tracheal tube was extubated, and the patient was transferred to the recovery room.
3.8. Follow-up and Data Collection
The senior resident, who performed all assessments, data collection, and follow-ups, was unaware of patients’ grouping (blinding). After transferring patients to the recovery room, if the patient was conscious enough to express pain after surgery, the pain intensity assessment was started using the visual pain criterion as previously instructed. Otherwise, pain assessment would be delayed until the patient regained full consciousness. Narcotic use was determined in two groups within 24 hours after surgery. The patients’ pain intensity was assessed using the Visual Analogue Scale (VAS) (0 for no pain and 10 for the most severe pain). During recovery based on pain intensity (VAS score > 3), if necessary, following the patient's first request for analgesia, meperidine at a dose of 1 mg/kg of body weight was injected by a nurse who was unaware (blind) of the study process. If pain was not relieved within half an hour, meperidine was repeated at the same dose. If there were postoperative nausea and vomiting, the patient received 4 mg ondansetron. Pain intensity at 3, 6, 12, and 24 hours after surgery, upper limb paresthesia, frequency of nausea and vomiting, shortness of breath, hematoma, and the length of hospital stay were also assessed.
3.9. Statistical Analysis
Data were analyzed using SPSS version 23 software (SPSS Inc. Chicago, The USA). A chi-square test was used to compare qualitative variables. Besides, quantitative variables were compared by the t test if they followed a normal distribution or the Mann-Whitney test if they did not have a normal distribution. Pearson's or Spearman’s correlation test was used to examining the correlation between variables. A P value of less than 0.05 was considered statistically significant.
4. Results
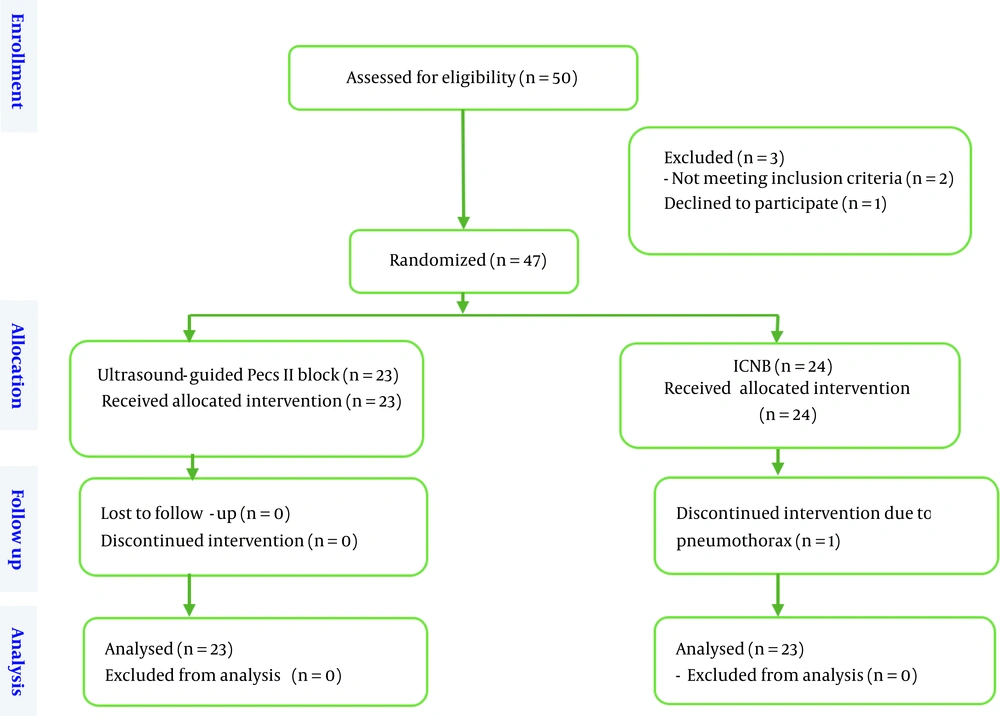
Fifty patients were included in the present clinical trial. Two patients were excluded from the study due to obesity and body mass index (BMI) above 35, and one patient was excluded due to dissatisfaction with the intervention. Finally, 47 candidates for mastectomy were included in the study and randomly divided into two groups (n = 23 under ultrasound-guided Pecs II block and n = 24 under ICNB) (Figure 1). In the ICNB group, one patient suddenly experienced a decrease in blood oxygen saturation and an increase in airway pressure after the block at the end of the operation. Due to bilateral pneumothorax, necessary measures were taken and the patient was excluded from the study. Bilateral chest tubes were placed, and the patient was admitted to the intensive care unit.
The mean age of patients in the group A was 30.54 ± 4.08, and in the group B was 29.46 ± 4.56 years, without a significant difference between them (P-value = 0.384)). The mean BMI of patients in the two groups of ultrasound-guided Pecs II block and ICNB was 25.08 ± 1.53 kg/m2 and 28.56 ± 4.44 kg/m2, respectively, without a significant difference (P-value = 0.712). The mean duration of surgery in the two groups of ultrasound-guided Pecs II block and ICNB was 256.96 ± 14.45 and 258.50 ±14.48 min, respectively, without a significant difference (P-value = 0.708). The mean duration of recovery after surgery in the two groups of ultrasound-guided Pecs II block and ICNB was 2.75 ± 0.94 h and 2.92 ± 1.06 h, respectively, without a significant difference (P-value = 0.545). The mean duration of total hospitalization stays in the two groups of ultrasound-guided Pecs II block, and ICNB was 2.08 ± 1.50 and 4.62 ± 1.60 days, respectively, with a statistically significant difference (P-value < 0.001). The mean dose of meperidine in the first 24 hours after surgery in the two groups of ultrasound-guided Pecs II block and ICNB was 13.33 ± 1.27 mg and 30.73 ± 1.71 mg, respectively, with a statistically significant difference (P-value < 0.001) (Table 1). The frequencies of postoperative nausea and vomiting in the two groups of ultrasound-guided Pecs II block and ICNB were three cases (13%) and 11 cases (47.8%), respectively, with a statistically significant difference (P-value = 0.019).
| Variable | Ultrasound-Guided Pecs II Block (N = 23) | ICNB (N = 23) | P-Value |
|---|---|---|---|
| Age (y) | 30.54 ± 4.08 | 29.46 ± 4.56 | 0.384 b |
| BMI (kg/m2) | 25.08 ± 1.53 | 28.56 ± 4.44 | 0.712 |
| Duration of surgery (min) | 256.9 ± 14.45 | 258.50 ± 14.48 | 0.708 |
| Duration of recovery (h) | 2.75 ± 0.94 | 2.92 ± 1.06 | 0.545 |
| Duration of hospitalization (d) | 2.08 ± 1.50 | 4.62 ± 1.60 | < 0.001 |
| Dosage of meperidine (mg) | 13.33 ± 1.27 | 30.73 ± 1.71 | < 0.001 |
Abbreviations: BMI, body mass index; ICNB, intercostal nerve block; Ultrasound-guided Pecs II block, ultrasound-guided type-II pectoral nerve block; SD, standard deviation.
aData are presented as mean ± standard deviation.
bt-test.
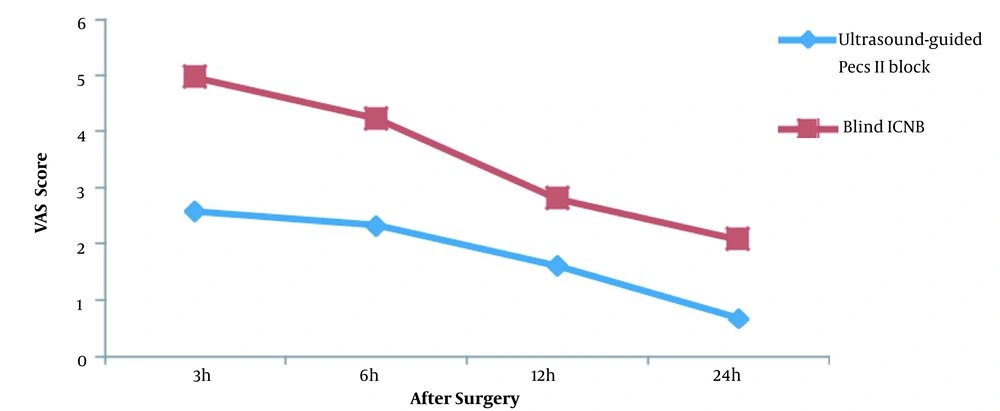
The mean pain scores at 3, 6, 12, and 24 hours after surgery were 2.58 ± 1.01, 2.33 ± 0.86, 1.62 ± 0.64, and 0.67 ± 0.56 in the ultrasound-guided Pecs II block group and 4.96 ± 1.39, 4.23 ± 1.14, 2.81 ± 0.80, and 2.08 ± 0.84 in the ICNB group, respectively. The trend of pain score changes in the two groups was statistically different (P-value < 0.001) (Table 2 and Figure 2).
| Time After Surgery | VAS | ||
|---|---|---|---|
| After Ultrasound-Guided Pecs II Block (N = 23) | ICNB (N = 23) | P-Value | |
| 3 h | 2.58 ± 1.01 | 4.96 ± 1.39 | 0.03 |
| 6 h | 2.33 ± 0.86 | 4.23 ± 1.14 | 0.015 |
| 12 h | 1.62 ± 0.64 | 2.81 ± 0.80 | 0.014 |
| 24 h | 0.67 ± 0.56 | 2.08 ± 0.84 | < 0.001 |
Abbreviations: ICNB, intercostal nerve block; Ultrasound-guided Pecs II block, ultrasound-guided type-II pectoral nerve block; VAS, Visual Analogue Scale.
aData are presented as mean ± standard deviation.
5. Discussion
According to the results of the study, which compared the two methods of ultrasound-guided Pecs II block with blind ICNB for pain control and surgical outcomes after breast tissue reconstruction surgery in transgender patients, the ultrasound-guided Pecs II block provided a better postoperative pain control, less opioid consumption, lower incidence of nausea and vomiting, and shorter length of hospital stay than ICNB while the recovery time was similar with both methods of nerve block.
Moreover, in the group where the nerve block was performed blindly by the surgeon (i.e., ICNB), one patient developed bilateral pneumothorax after the block. Therefore, the use of ultrasound guidance in pectoral nerve tracking to block the nerve would lead to better outcomes and fewer complications than nerve tracking by the surgeon. There are several studies in the literature on the use of ultrasound guidance for blocking the pectoral nerve, but there are limited studies showing the superiority of this method over nerve tracking without ultrasound guidance, as well as its more efficiency.
In the study by Mansour et al., the group under ultrasound guidance had a lower catheter-visibility score, shorter block application time, and less effort to block than the group under standard nerve blocking (40). The results of this study are in line with our study. In the study by Wijayasinghe on the evaluation of the nerve using ultrasound, only the second intercostal space was visible using ultrasound, which was sufficient to perform a nerve block. Also, the use of sonography in tracking the pectoral nerve pathway was associated with a change in the pain score of about 9 units, as a significant reduction (39). In the study by Wang et al., a significant reduction in intraoperative morphine and fentanyl consumption was reported in the group under ultrasound guidance (41), which is in line with the present study. In the study by Lovett-Carter et al. in 2019, a systematic review was conducted on clinical studies evaluating the effectiveness of pectoral block in patients undergoing mastectomy. In this evaluation, the intensity of postoperative pain within the first six hours was significantly lower in the group under nerve block with ultrasound guidance than in the control group (42). However, in the study by Sun et al., the use of pectoral nerve block under ultrasound guidance was significantly associated with a reduction in pain intensity during hospitalization, as well as 24 hours after surgery. The nerve block was also associated with decreased opioid use, but no change was made in the rate of postoperative complications, including nausea and vomiting (43). Finally, in the study by Zhao et al., patients undergoing pectoral nerve block II under ultrasound guidance had a significantly reduced intraoperative and postoperative opioid use, postoperative nausea and vomiting, and postoperative pain within six hours after surgery (44). These findings are consistent with the present study. Therefore, due to the effect of pectoral nerve blocking, especially with ultrasound guidance, on reducing postoperative pain, the dose of opioids, complications, and also the total length of hospital stay, the use of ultrasound in nerve tracking can be routinely considered after mastectomies.
To our knowledge, there is no study available in the literature regarding pain reduction techniques after reconstruction breast surgery in transgender patients. Also, in most studies, the pectoral nerve block was done immediately after the induction of anesthesia or before the induction of anesthesia when the patient was awake, but in our study, we performed it at the end of the operation, which could affect the duration of analgesia after surgery. Since these people are more prone to more psychological damage depending on how they are perceived and treated in society, they will require more support indeed (38).
In this study, the first and main limitation was the occurrence of the COVID-19 pandemic, thereby reducing the number of patients referring to medical centers, which consequently reduced the number of operations to some extent. The next limitation was the underlying cultural conditions for transgender patients. The exclusion of such people from the family interferes with the diagnosis process so that the number of cases referred to the hospital is fewer than the actual number of cases in the community. On the other hand, due to the high expenses of several stages of operations and hospitalization in medical centers, a high percentage of patients are not able to perform all stages, and therefore completing the treatment process is difficult (38). It also needs to be mentioned that the two types of blocks in this study were not performed at a similar time; one was performed a few minutes before the end of the surgery, and the other was performed at the end of surgery, which may have decreased the accuracy of the comparison performed. In addition, it was better to use the same interventional methods for both procedures, but due to the unavailability of ultrasound in many institutes, our study aimed to compare a conventional, less equipment-intensive method of nerve block with an ultrasound-guided one.
Due to the mentioned limitations and difficult conditions, it is recommended to perform studies with a larger sample size. Moreover, it is recommended to perform studies to compare postoperative pain in transgender patients and those with breast cancer. Also, financial aid from social affairs can facilitate the diagnosis and accomplishment of surgical correction, which, therefore, could improve the psychosocial conditions of these patients.
5.1. Conclusions
The pectoral nerve block under ultrasound guidance, compared to blind ICNB, in breast tissue reconstruction of transgender patients can reduce pain intensity and the required dosage of opioids within 24 hours, the incidence of postoperative nausea, vomiting, and hospital stay.