1. Background
Coronary artery bypass grafting (CABG) is a common therapeutic intervention with several postoperative complications, including electrolyte disturbances. Electrolyte disturbances are key factors in the occurrence of cardiac arrhythmias; therefore, it is essential to check serum electrolytes several times in the first 24 hours and at least once a day thereafter to prevent them. According to studies, the most common postoperative electrolyte disturbances are potassium abnormalities (1-3).
Potassium can affect the activity of skeletal and cardiac muscles. Any change in potassium concentration changes the excitability and rhythm of the cardiac muscle. Average potassium concentration varies within 3.5 - 5 mEq/L according to the needs of the body and under the influence of the sodium-potassium pump (4, 5).
Another common complication after CABG is the occurrence of supraventricular arrhythmias, reported up to 40%. Although supraventricular arrhythmias are benign and controllable, they can lead to dangerous complications, such as stroke. Electrolyte imbalance plays a role in postoperative arrhythmias. Moreover, to prevent the occurrence of these arrhythmias, serum electrolytes should be measured regularly after the operation, checking for hyper/hypokalemia, hypomagnesemia, hyponatremia, and hypocalcemia (6-8).
Problems that occur in the early hours after CABG are acute and critical. Various risk factors affect the mortality rate and postoperative complications (9). Old age, gender, emergency surgery, preoperative myocardial infarction, cardiac output, ejection fraction (EF), number of affected vessels, underlying diseases, previous surgery, history of heart failure, history of lung disease, surgeon skills, duration of cardiac and respiratory pump use, body mass index (BMI), smoking, duration of surgery, duration of aortic occlusion, intraoperative temperature, duration of mechanical ventilation after surgery, and administration of cardiovascular and vasoactive drugs are some of the factors associated with postoperative electrolyte disorders (10-16).
The prevalence of cardiovascular diseases, especially coronary artery disease, is high. Furthermore, the most common therapeutic intervention in these patients is CABG. Moreover, there is a variety and distribution of complications and differences in their occurrence in patients undergoing CABG in several studies. Due to the aforementioned three factors, this study was designed to determine the extent of potassium disorders in patients undergoing CABG.
2. Objectives
This study aimed to investigate the relationship between potassium concentration and cardiac arrhythmias in CABG surgery.
3. Methods
This descriptive cross-sectional study was performed on 60 patients undergoing CABG in Golestan Hospital, Ahvaz, Iran, after obtaining the necessary permits from the Ethics Committee of Anesthesiology and Pain Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (IR.AJUMS.HGOLESTAN.REC.1399.060). The inclusion criteria consisted of patients scheduled for elective CABG surgery, the American Society of Anesthesiologists (ASA) class I, II, or III, absence of significant renal and hepatic problems, absence of any arrhythmia on the last electrocardiogram (ECG), and BMI less than 35 kg/m2. Patients with known electrolyte disorders before the surgery, known arrhythmias, need for emergent interventions, and an EF of less than 35% were excluded from the study.
After obtaining the approval of the proposal, permission from the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences, and informed consent from patients who were candidates for CABG surgery, all eligible patients entered the study. The procedure was explained to the patients, and then the patients were visited by a cardiac surgery anesthesia fellowship. After the establishment of a safe line for surgery, the patients were transferred to the operating room. All patients with ASA class I, II, or III were included in the study.
All patients received 7 cc/kg of normal saline before induction. In preoperative evaluations, age, gender, BMI, smoking status, medical history of cardiovascular diseases (including hypertension, atrial fibrillation, congestive heart failure, and previous ischemic heart disease or CABG intervention), antihypertensive medication (eg, angiotensin-converting enzyme inhibitors, beta blockers, and calcium channel blockers), and history of insulin-dependent gestational diabetes (in female patients) were reassessed. Vital signs were assessed by ECG monitoring, pulse oximetry, and invasive blood pressure measurement through the arterial catheter during surgery. Intraoperative events, including hypotension (30% reduction in baseline mean arterial pressure [MAP] for longer than 5 minutes), MAP < 60 mmHg, and tachycardia (increase in the heart rate more than 30 bpm from the baseline heart rate for longer than 5 minutes), were recorded by anesthesia charts. Intraoperative bleeding volume (mL) and fluid balance during the first 24 hours after surgery were assessed to ensure basal blood pressure.
All patients underwent general anesthesia by a similar method (midazolam 0.25 mg/kg, fentanyl 3 μg/kg, etomidate 0.4 mg/kg, and cisatracurium 0.25 mg/kg). Anesthesia was maintained with midazolam 0.15 mg/kg/h, fentanyl 6 μg/kg/h, and cisatracurium 0.25 mg/kg/h infusion. Ventilation control was achieved using a mechanical ventilator maintained at 35 - 45 mmHg carbon dioxide pressure. The blood samples were taken for blood gas analysis and laboratory tests for potassium, sodium, magnesium, calcium, blood urea nitrogen (BUN), creatinine, and blood sugar levels at different time points, including before induction of anesthesia, after induction of anesthesia, before cardiopulmonary bypass (CPB), on CPB, after separation from CPB, and on entering the intensive care unit (ICU).
The CPB circuit was primed with glucose-free crystalloid solutions (ie, lactated Ringer’s solution, mannitol, and Voluven). The pump flow rate (with the direct flow and without pulse) was set at 2 - 2.4 L/min/m2 according to the patient’s temperature and recorded continuously. After starting CPB, the patient was cooled to 32 - 34°C depending on the type of surgery and operating conditions. Membrane oxygenators and arterial filters were used during surgery. Del Nido cold cardioplegia solutions were used antegradely or retrogradely to establish chemical arrest before the implantation of the aortic clamp. Alpha acetate strategy was used in all patients to regulate arterial partial pressure of carbon dioxide at normal levels. During CPB, anesthesia was continued with continuous injections of anesthetics. In all patients, an attempt was made to maintain the hematocrit of patients above 24 to 27%; for this purpose, blood was transfused if necessary (17). For this study, surgeons who used del Nido cardioplegia and had similar routines were used.
The blood samples were taken for blood gas analysis and laboratory tests for potassium, sodium, magnesium, calcium, BUN, creatinine, and blood sugar levels at different time points, including before induction of anesthesia, after induction of anesthesia, before CPB, on CPB, after separation from CPB, and on entering the ICU.
3.1. Primary Outcomes
All cardiac arrhythmias were recorded at the above-mentioned time points (ie, before induction of anesthesia, after induction of anesthesia, before CPB, on CPB, after separation from CPB, and on entering the ICU).
3.2. Secondary Outcomes
The levels of potassium, MAP, serum level of potassium, blood sugar, BUN, creatinine, calcium, magnesium, hemoglobin (Hb), and sodium were recorded.
3.3. Sample Size Calculation
The sample size was calculated using the sample size formula consisting of 58 patients, according to the significance level of 0.05 and the power of 0.8. The sample size was increased to 60 to improve the measurement accuracy.
3.4. Statistical Analysis
For quantitative variables, mean (and/or median) was used to describe the data center, and standard deviation (and/or interquartile range) was used to describe data scatter. Frequency and percentage were used for the description of qualitative variables. The Chi-square test (or Fisher’s exact test) and t-test (or Mann-Whitney U test) were used as necessary to analyze the data. P-values less than 0.05 were considered statistically significant. All analyzes were performed using SPSS software (version 22).
4. Results
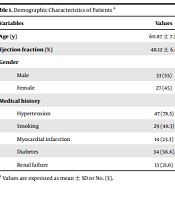
Table 1 shows the demographic information of patients. Based on the results, the mean age of patients was 60.87 ± 7.35 years, with a mean EF of 48.12 ± 6.5. Furthermore, 33 (55%) and 27 (45%) patients were male and female, respectively. There was no significant difference between the two arrhythmic and nonarrhythmic groups in terms of demographic variables (P > 0.05).
| Variables | Values |
|---|---|
| Age (y) | 60.87 ± 7.35 |
| Ejection fraction (%) | 48.12 ± 6.5 |
| Gender | |
| Male | 33 (55) |
| Female | 27 (45) |
| Medical history | |
| Hypertension | 47 (78.3) |
| Smoking | 29 (48.3) |
| Myocardial infarction | 14 (23.3) |
| Diabetes | 34 (56.6) |
| Renal failure | 13 (21.6) |
Demographic Characteristics of Patients a
The trend of changes in potassium levels at different time points showed a significant difference (P = 0.045; Table 2). Table 2 shows the trend of changes in MAP, other electrolyte levels (ie, sodium, calcium, magnesium, and BUN/creatinine), and blood sugar in all patients. According to the analysis, the changes in MAP, blood sugar, calcium, magnesium, and BUN/creatinine at different time points showed a significant difference (P < 0.05). However, the trend of changes in sodium and Hb levels did not show a significant difference (P > 0.05).
| Variables | Before Operation | After Induction of Anesthesia | On Cardiopulmonary Bypass | After Cardiopulmonary Bypass | On Entering the Intensive Care Unit | P-Value a |
|---|---|---|---|---|---|---|
| Mean arterial pressure (mmHg) | 85.0 ± 4.6 | 85.6 ± 6.2 | 77.5 ± 5.9 | 75.0 ± 4.6 | 80.0 ± 5.3 | 0.003 |
| Blood urea nitrogen (mg/dL) | 18.0 ± 4.5 | 17.4 ± 4.6 | 17.2 ± 4.3 | 18.4 ± 2.1 | 18.3 ± 4.0 | 0.001 |
| Creatinine (mg/dL) | 1.1 ± 0.2 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.09 | 1.2 ± 0.2 | 0.002 |
| Hemoglobin (g/dL) | 13.1 ± 1.6 | 10.7 ± 2.5 | 9.6 ± 1.9 | 9.9 ± 2.1 | 10.3 ± 1.7 | 0.052 |
| Potassium (mmol/L) | 4.3 ± 0.5 | 4.6 ± 1 | 5.5 ± 1.2 | 5.4 ± 0.8 | 4.7 ± 0.6 | 0.045 |
| Sodium (mEq/L) | 140.7 ± 4.8 | 140.4 ± 4.7 | 139.6 ± 3.2 | 139.1 ± 4.6 | 139.8 ± 5.3 | 0.905 |
| Blood sugar (mg/dL) | 118.7 ± 51.9 | 128.5 ± 71.5 | 149.2 ± 87.8 | 177.8 ± 72.3 | 166.3 ± 65.8 | 0.032 |
| Calcium (mg/dL) | 8.5 ± 1.13 | 8.5 ± 0.6 | 8.2 ± 0.3 | 8.3 ± 0.3 | 8.2 ± 0.5 | < 0.001 |
| Magnesium (mg/dL) | 2.15 ± 0.4 | 2.4 ± 0.4 | 2.5 ± 0.3 | 2.3 ± 0.4 | 2.4 ± 0.3 | 0.001 |
Trends of Studied Factors at Different Measurement Time Points
In the analyses of two arrhythmic and nonarrhythmic groups, a significant difference was observed between the two groups in the potassium level (P < 0.001; repeated measures analysis of variance). The other variables, such as MAP, BUN, creatinine clearance, Hb, sodium, blood sugar, calcium, and magnesium plasma levels, were not significantly different (Figure 1). Table 3 shows the prevalence of arrhythmias at different time points. According to the presented results, the highest and lowest incidence of arrhythmia was after aortic cannulation and after skin incision, respectively.
(A) Changes in mean arterial pressure (MAP) in arrhythmic group (n = 27) and nonarrhythmic group (n = 33) before the operation, after induction, on pump, after pump, and on entering to intensive care unit measurements (P = 0.082; repeated measures analysis of variance [ANOVA]); (B) Changes in blood urea nitrogen in arrhythmic group (n = 27) and nonarrhythmic group (n = 33) (P < 0.005; repeated measures ANOVA); (C) Changes in creatinine clearance (P = 0.519; repeated measures ANOVA); (D) Changes in hemoglobin (P = 0.486; repeated measures ANOVA); (E) Changes in blood potassium level (P < 0.001; repeated measures ANOVA); (F) Changes in blood sodium level (P > 0.0.5; repeated measures ANOVA); (G) Changes in blood sugar level (P > 0.0.5; repeated measures ANOVA); (H) Changes in blood calcium level (P > 0.0.5; repeated measures ANOVA); (I) Changes in blood magnesium level (P > 0.0.5; repeated measures ANOVA)
| Time of Disorder | No. (%) |
|---|---|
| Antiarrhythmic administration | 17 (28.3) |
| After induction of anesthesia | 13 (21.6) |
| Arrhythmia after skin incision | 9 (15) |
| Incidence of arrhythmia after sternotomy | 15 (25) |
| Incidence of arrhythmia after aortic cannulation | 27 (45) |
| Incidence of arrhythmia after right atrial cannulation | 19 (31.6) |
| Arrhythmia in the first minutes of separation from the cardiopulmonary bypass | 21 (35) |
| Incidence of bradyarrhythmia | 11 (40.7) |
| Incidence of tachyarrhythmia | 16 (59.3) |
Frequency of Arrhythmia at Different Time Points
5. Discussion
The findings of this study suggest that the changes in MAP, potassium, calcium, magnesium, BUN/creatinine, and blood sugar but not sodium and Hb had a role in the occurrence of cardiac arrhythmias (17). In a 2017 study conducted by Patel et al., serum preoperative potassium levels, cardiac outcomes, and mortality rates were examined in 6,515 patients with acute coronary syndrome. The results showed that the risk of cardiac arrhythmias, such as paroxysmal supraventricular tachycardia, was 4.5% higher in serum potassium levels ≤ 3.5 mEq/L (P = 0.03). The probability of mortality and U-wave formation in patients with hypokalemia was 2% and 3% higher than in other patients, respectively (P = 0.01). The authors concluded that abnormal potassium levels are associated with increased risks of tachyarrhythmias and death (18). In the present study, the changes in potassium levels were significantly associated with the occurrence of cardiac arrhythmias and are consistent with the results of the aforementioned study.
A 2016 study performed by Uluganyan et al. examined the relationship between potassium levels in patients with acute coronary syndrome and the incidence of arrhythmias and mortality in 277 patients with myocardial infarction within 2010-2013. This study showed that patients with high potassium (≥ 5.2 mEq/L) experienced a higher incidence of cardiac arrhythmias and mortality and reported a significant relationship between potassium levels less than 3.5 and more than 5 and the incidence of ventricular arrhythmias (19, 20). In 2015, Peng et al. examined the association between serum potassium levels and the incidence of arrhythmias and mortality in patients undergoing coronary angiography. They showed that the incidence of arrhythmias and mortality in patients with potassium levels less than 3.5 and more than 5 mEq/L was significantly higher than in the other groups (21). The results of the present study are consistent with the results of both aforementioned studies.
In another study, Chio et al. examined the association between serum potassium levels and the incidence of arrhythmias and other cardiac outcomes. The results showed that the probability of arrhythmias in patients with potassium levels of more than 4.5 and less than 3.5 is more than other patients. They concluded that potassium levels are effective in the development of ventricular arrhythmias; however, there was no significant relationship between the level of potassium and long-term mortality (22).
A study performed by Krijthe et al. on the relationship between serum potassium levels and the incidence of atrial fibrillation in 2013 showed that patients with hypokalemia have a higher risk of atrial fibrillation, and serum potassium levels are associated with an increased risk of atrial fibrillation (23).
The current study also investigated the relationship between the levels of calcium, phosphorus, magnesium, blood sugar, Hb, and BUN/creatinine, with the incidence of cardiac arrhythmias in this population. There was a significant relationship between the levels of calcium, magnesium, BUN, creatinine, and MAP with the incidence of arrhythmias; nevertheless, no significant relationship was observed between the changes in the levels of sodium and Hb and the incidence of cardiac arrhythmias.
Contrary to the findings of the present study, Zeighami Mohammadi and Asgharzadeh Haghighi in 2011 reported hyponatremia as the most common (16.2%) electrolyte disturbance in patients hospitalized due to heart failure (24). The results of the present study are similar to the results of a study by Gheorghiade et al. in 2007, indicating that 25.3% of 47,647 patients with heart failure had hyponatremia at the time of admission (25). This difference in the results can be related to the use of diuretics, the difference in renin-angiotensin-aldosterone activity, or the presence of hyperglycemia in patients with heart failure.
The results of a 2016 meta-analysis conducted by Angkananard et al. on the relationship between serum magnesium levels and mortality in heart failure patients showed that hypomagnesemia significantly increased mortality due to myocardial infarction, which is in line with the findings of the present study (26). The current study on serum calcium levels in cardiac patients showed notable differences and similarities in comparison to the results of previous studies. Mousavi Movahed et al. observed no significant relationship between serum calcium levels with heart disease and the calcification of heart valves (27). However, the results of studies performed by Zand Parsa et al. (28) and Nematollahi et al. (29) showed a direct linear relationship between calcium levels and the severity of heart disease in these patients. The difference in sample size in these two studies could cause this discrepancy in the results. Zeighami Mohammadi and Asgharzadeh Haghighi stated that in heart failure patients with some degree of renal impairment and higher BUN/creatinine levels, the severity of the disease and its complications are more severe (24), which is consistent with the results of the present study.
5.1. Conclusions
According to the results of this study, it can be concluded that the changes in potassium levels increase the risk of cardiac arrhythmias and their complications.
5.2. Limitations
Among the limitations of the present study are the limited sample size and laboratory limitations to investigate the factors that require further studies with more samples.
![(A) Changes in mean arterial pressure (MAP) in arrhythmic group (n = 27) and nonarrhythmic group (n = 33) before the operation, after induction, on pump, after pump, and on entering to intensive care unit measurements (P = 0.082; repeated measures analysis of variance [ANOVA]); (B) Changes in blood urea nitrogen in arrhythmic group (n = 27) and nonarrhythmic group (n = 33) (P < 0.005; repeated measures ANOVA); (C) Changes in creatinine clearance (P = 0.519; repeated measures ANOVA); (D) Changes in hemoglobin (P = 0.486; repeated measures ANOVA); (E) Changes in blood potassium level (P < 0.001; repeated measures ANOVA); (F) Changes in blood sodium level (P > 0.0.5; repeated measures ANOVA); (G) Changes in blood sugar level (P > 0.0.5; repeated measures ANOVA); (H) Changes in blood calcium level (P > 0.0.5; repeated measures ANOVA); (I) Changes in blood magnesium level (P > 0.0.5; repeated measures ANOVA) (A) Changes in mean arterial pressure (MAP) in arrhythmic group (n = 27) and nonarrhythmic group (n = 33) before the operation, after induction, on pump, after pump, and on entering to intensive care unit measurements (P = 0.082; repeated measures analysis of variance [ANOVA]); (B) Changes in blood urea nitrogen in arrhythmic group (n = 27) and nonarrhythmic group (n = 33) (P < 0.005; repeated measures ANOVA); (C) Changes in creatinine clearance (P = 0.519; repeated measures ANOVA); (D) Changes in hemoglobin (P = 0.486; repeated measures ANOVA); (E) Changes in blood potassium level (P < 0.001; repeated measures ANOVA); (F) Changes in blood sodium level (P > 0.0.5; repeated measures ANOVA); (G) Changes in blood sugar level (P > 0.0.5; repeated measures ANOVA); (H) Changes in blood calcium level (P > 0.0.5; repeated measures ANOVA); (I) Changes in blood magnesium level (P > 0.0.5; repeated measures ANOVA)](https://services.brieflands.com/cdn/serve/315dc/27fcf9b4f779fd28c6cf9f93bb6b560da99b935c/aapm-12-1-121809-i001-preview.png)