1. Background
Cesarean section is one of the most common surgeries in the world, which has been increased in the last 21 years (1). Spinal anesthesia is the method of choice for elective and emergency Cesarean sections. Simple technique, fast efficacy, and uniform sensory and motor blocks are the advantages of spinal anesthesia. Also, short-term duration and lack of prolonged postoperative analgesia can be mentioned as the probable disadvantages (2). Injected neuroaxial drugs influence their surrounding tissues by the penetration. To date, many drugs have been used, but local anesthetics are the most important drugs (3-5). Bupivacaine at a concentration of 0.5%, became popular and a drug of choice for Cesarean section because of its long term block, sensory block separated from the motor block, relative lack of tachyphylaxis, and limited placental transfer (3). To increase the duration of anesthesia and improve the quality of analgesia during and after surgery, intrathecal opioids are used in combination with local anesthetics. It seems that opioid drugs in combination with local anesthetics have synergistic effects in spinal anesthesia (2, 3, 5). Hunt et al. (4) described intrathecal fentanyl as a lipophilic opioid for the first time. Previous investigations indicated that by adding fentanyl or more lipophilic drugs such as sufentanil to bupivacaine for spinal anesthesia, analgesia during and after surgery will be improved (5-7).
2. Objectives
Previous investigations assessed different opioids and mentioned diverse results by adding opioids to local anesthetics to reduce the dosage of local anesthetics, but increase in duration of analgesia and their effects on hemodynamic status and complications proved them to be safe for Cesarean section. It is well known that age, sex, ethnicity, race, and pregnancy are important factors, which can alter the patient’s response to analgesic drugs (8). To the best of our knowledge, most of the previous Iranian investigations assessed the effects of subarachnoid injection of opioids in combination with local anesthetics on orthopedic surgeries in diverse age groups and on both sexes (9). Also, previous investigations on pregnant women revealed that different drugs and dosages were administered to these women and most of them received local anesthetic lidocaine for spinal anesthesia (10). However, recently its usage has been limited due to the neurologic complications, and bupivacaine has been introduced as the first choice of local anesthetic for spinal anesthesia.
Considering the safety of bupivacaine in pregnancy, ethnic differences, and lack of similar researches in our country during pregnancy, and the importance of anesthesia in this group and its controversial concern for anesthesiologists, in this study, we aimed at comparing the effect of adding fentanyl, sufentanil, and placebo to intrathecal bupivacaine on duration of analgesia and complications of spinal anesthesia in patients undergoing Cesarean section.
3. Methods
This was a double-blind clinical trial which was conducted in Anesthesiology Research Center of Guilan University of Medical Sciences. The ethics committee of Guilan University of Medical Sciences approved this study (IR.GUMS.REC.1394.23), with the following IRCT code: IRCT201506068677N6.
Inclusion criteria were pregnant women, who were candidates for elective Cesarean section with the American Society of Anesthesiologists Class I-II, aged 17 to 45 years, and 150 to 170 cm height, without any history of addiction or spinal anesthesia contraindication such as high intracranial pressure, coagulopathy, skin infection at the injection site, allergy to opioids or local anesthetics, and morbid obesity.
Exclusion criteria were concomitant multiple surgeries, surgery duration more than 90 minutes, volume of bleeding more than 1500 cc during the surgery, and lack of sufficient sensory level for Cesarean section, and the need for general anesthesia.
Sample size was determined according to the following formula and derived from the study by Nesionpoor et al. (10).
α = 0.05
β = 0.10
z1-α/2 = 1.96
z1-β = 0.84
S1 = 2.3
S2 = 4.2
D = 2.4

With the probable drop rate of 10%, the sample size of 33 patients was determined for each group.
Using the method of random blocking, patients were divided into 3 groups (fentanyl: F group, sufentanil: S group, and placebo: P group), with 33 patients in each group. The day before surgery, the type of surgery, anesthesia type, and the method of assessment during and after surgery were explained at the bedside for patients, and an informed written consent was obtained from them. However, the researcher and the patients were unaware of the type of chosen drug. At the operating room, all patients underwent standard monitoring including ECG, HR, NIBP, and pulse oximetry (SAADAT Digital Monitoring). After inserting an 18 gauge intravenous cannula, 10 mL/kg normal saline solution was injected during 15 to 30 minutes, then, spinal anesthesia was performed in sitting position by a skilled anesthesiologist (> 10 years of experience) using a 25-guage Quinke needle (B.Brown Company) through L3-L4 or L4-L5 intervertebral space.
For blinding, the anesthesiologist who performed the neuroaxial blocking prepared the drugs to assess complications and perform necessary procedures if needed. Patient and the investigators were unaware of the types of drugs.
For group F, 12.5 mg of bupivacaine and 25 mcg of fentanyl, for group S, 12.5 mg of bupivacaine and 2.5 mcg of sufentanil, and for group P, 12.5 mg of bupivacaine and a half mL of normal saline were injected.
Injection volume in all patients was 3 mL. Bupivacaine, fentanyl, and sufentanil were produced by Astra Zeneca, Abu Reyhan and, Janssen Companies, respectively. After spinal anesthesia, the patient was immediately placed in a supine position with left uterine displacement, and supplemental oxygen was administered via a face mask at a rate of 5 - 8 L/min. After the injection of local anesthetic, the sensory block, and maximum sensory block level were assessed with the patient’s ability to distinct the sharpness created by the tip of the needle (pin prick method) (11) and motor block level were measured by examining skeletal muscle strength criteria using modified Bromage scale (0 = no paralysis, 1 = only able to move the knee, 2 = only able to move feet, 3 = inability to move the leg or knee) (12). Also, pain was evaluated based on the visual analogue scale (VAS). In VAS scoring, 0 indicated no pain and 10 the most severe pain (13).
After spinal anesthesia, the level of sensory and motor block and analgesia were evaluated every 3 minutes for the first 15 minutes, every 15 minutes for the next 45 minutes, and every 30 minutes up to 6 hours. The duration between the end of intrathecal injection to decreased pinprink sense below T10 and the duration between the ends of intrathecal injection to free feet movement, respectively, indicated the durations of sensory and motor blocks. During the study, whenever patients reported pain with the VAS score ≥ 4, diclofenac suppository 50 mg was administered for them. Duration of analgesia was from the end of intrathecal injection to the occurrence of VAS score ≥ 4.
Baseline blood pressure and heart rate were measured before spinal anesthesia and after spinal anesthesia; blood pressure and heart rate were measured every 3 minutes before and every 5 minutes after childbirth. If systolic blood pressure was less than 90 mmHg, 5 mg intravenous ephedrine up to maximum dose of 30 mg, and in case of bradycardia (heart rate less than 60 beats/minutes), 0.5 mg intravenous atropine was administered. Immediately after neonatal delivery, 30 units of IV infusion oxytocin were administered within 1 hour.
All patients were assessed within 24 hours after surgery for analgesia and potential drug complications such as itching, drowsiness, respiratory depression, nausea, and vomiting. In case of nausea and vomiting, 0.1 mg/kg intravenous metoclopramide, in case of itching, first 25 mg intramuscular promethazine, in nonrespondents, 0.08 mg intravenous naloxone, and in case of respiratory depression (respiratory rate less than 9 per minute) first 0.08 mg intravenous naloxone, and in nonrespondents, 0.04 mg intravenous naloxone were injected. Sedation score was assessed based on the Ramsay sedation score: (1) anxious, agitated, restless; (2) tranquil, cooperative, oriented; (3) responsive to commands only; (4) brisk response to light glabellar tap or loud auditory stimulus; (5) sluggish response to light glabellar tap or loud auditory stimulus; (6) no response to light glabellar tap or loud auditory stimulus; and (14) the first and fifth minutes APGAR score were recorded.
3.1. Statistical Analysis
After data collection, data were entered into SPSS Version 17. Quantitative data were analyzed by ANOVA and Kruskal- Wallis test (post hoc Turkey), and qualitative data were analyzed by chi- square test.
4. Results
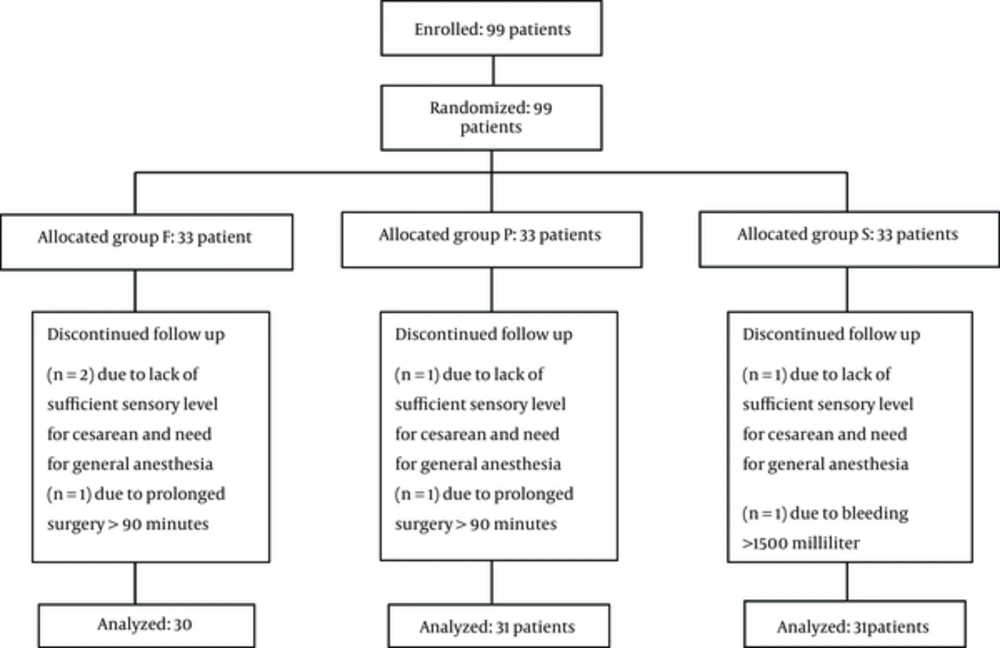
From 99 patients who entered the study, 7 were excluded. Therefore 31, 30, and 31 patients were enrolled in groups S, F, and P, respectively (Figure 1).
Patients’ demographic characteristics were similar in all 3 groups (Table 1). The durations of sensory block and motor block were higher in F and S groups compared with the P group (Table 2), and there were statistically significant differences among the 3 groups (P = 0.0001). Duration of analgesia had no difference between F and S groups, but in both groups it was more than the P group (P = 0.0001). The sensory and motor block and duration of analgesia analyses were performed using Kruskal-Wallis test.
| Variable | Group P | Group S | Group F | P Value |
|---|---|---|---|---|
| Age, y | 30.09 ± 4.45 | 29.67 ± 5.5 | 28.66 ± 5.77 | 0.556 |
| ASA Class | 0.333 | |||
| ClassI | 23 (74.2) | 19 (61.3) | 17 (56.7) | |
| ClassII | 8 (25.8) | 12 (38.7%) | 13 (43.3) | |
| BMI | 29.77 ± 3.07 | 30.47±2.85 | 31.01 ± 2.72 | 0.25 |
aValues are expressed as mean ± SD or No. (%).
| Variable | Group P | Group S | Group F | P Value |
|---|---|---|---|---|
| Duration of surgery, min | 50.32 ± 9.74 | 54.35 ± 17.3 | 49.66 ± 11.66 | 0.591 |
| Duration of sensory recovery to T10 level | 96.2 ± 32.35 | 150 ± 25.6 | 129 ± 19.53 | 0.0001 |
| Duration of motor block | 223 ± 45.05 | 320 ± 32.05 | 291 ± 17.87 | 0.0001 |
| Duration of analgesia | 116.1 ± 42.24 | 312.5 ± 34.44 | 314 ± 42.95 | 0.0001 |
The highest sensory level in all 3 groups was T4. Although more patients in F and S groups, compared with P group, had higher sensory and motor levels, the results of the Fisher’s exact test revealed no significant difference between the groups (Table 3).
| Variable | Group P | Group S | Group F | P Value |
|---|---|---|---|---|
| Sensory block level | P = 0.257 | |||
| T4 | 17 (54.8) | 25 (80.6) | 22 (73.3) | |
| T5 | 9 (29) | 5 (16.1) | 6 (20) | |
| T6 | 3 (9.7) | 1 (3.2) | 2 (6.7) | |
| T7 | 2 (6.5) | 0 | 0 | |
| Motor block degree | P = 0.369 | |||
| 0 | 0 | 0 | 0 | |
| 1 | 2 (6.5) | 0 | 0 | |
| 2 | 5 (16.1) | 4 (12.9) | 5 (16.7) | |
| 3 | 24 (77.4) | 27 (87.1) | 25 (83.3) |
aValues are expressed as No. (%).
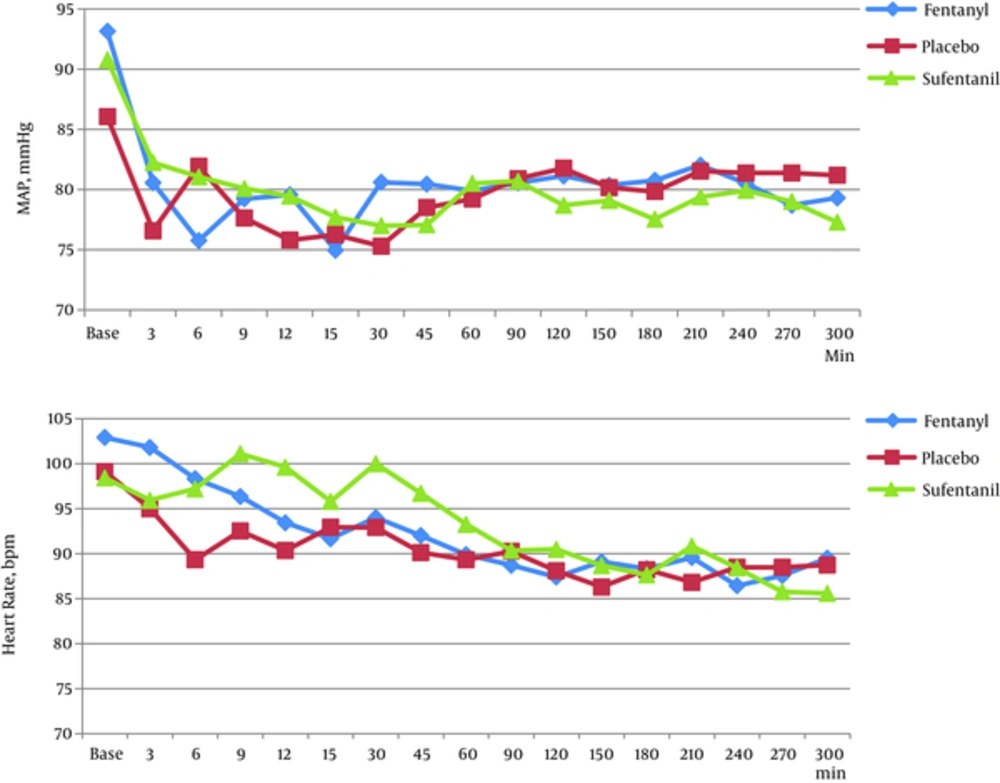
Considering the ANOVA test results, no significant difference was observed among groups in the hemodynamic parameters in all periods of assessments (BP, HR) (P > 0.05) (Figure 2). The frequency of itching in the F group was higher than S and P groups (P = 0.003). Also, shivering was higher in the P group compared with the 2 other groups (P = 0.036). However, no significant difference was obtained between groups in nausea, vomiting, and respiratory depression based on the Fisher’s, exact test (Table 4). A statistically significant difference was obtained in the incidence of sedation among groups (P = 0.019). Nevertheless, regarding the first and 5th minutes Apgar score, no significant difference was detected between the 3 groups (P > 0.05) based on the Kruskal- Wallis test (Table 4).
| Variable | Group P | Group S | Group F | P Value |
|---|---|---|---|---|
| Complications | ||||
| Nausea | 3 (9.7) | 1 (3.2) | 2 (6.7) | 0.69 |
| vomiting | 2 (6.5) | 0 (0) | 0 (0) | 0.326 |
| Itching | 0 (0) | 0 (0) | 5 (16.7) | 0.003 |
| Shivering | 11 (35.5) | 5 (16.1) | 3 (10) | 0.036 |
| Sedation score | ||||
| 1 (restless) | 9 (29) | 1 (3.2) | 3 (10) | 0.019 |
| 2 (cooperative) | 20 (64.5) | 29 (93.5) | 23 (76.7) | |
| 3 (Responsive to commands only) | 2 (6.5) | 1 (3.2) | 4 (13.3) | |
| 4 ( Brisk response to light glabellar tap) | 0 | 0 | 0 | - |
| 5 (Sluggish response to light glabellar tap) | 0 | 0 | 0 | - |
| 6 (No response to light glabellar tap) | 0 | 0 | 0 | - |
| Apgar | ||||
| First min | 8.03±0.48 | 7.9±0.3 | 7.93±0.25 | 0.36 |
| Fifth min | 9.09±0.53 | 9±0.36 | 8.96±0.18 | 0.18 |
aValues are expressed as No. (%).
5. Discussion
Nowadays, spinal anesthesia is the method of choice for most elective and emergency Cesarean sections. Bupivacaine is the choice for local anesthetic drug in Cesarean section, which affects through blocking the voltage gate sodium channels (15). Recently, to improve the analgesic quality, increasing the duration of anesthesia and reducing the dose of local anesthetics, many drugs such as magnesium sulfate, midazolam, dexmedetomidine, dexamethasone, and opioids in combination with local anesthetics have been commonly used for neuroaxial blocks (11-14, 16-20). Among opioids, lipophilic drugs (fentanyl, sufentanil) have appropriate pharmacological effects for spinal anesthesia. These drugs have rapid onset, moderate duration of action, and low affinity to expand to the fourth vertebrae, and thus they are associated with a decreased risk of respiratory depression (21). Regardless of the wide use of opioids (6.25 - 50 mcg of fentanyl and 2.5 - 7.5 mcg of sufentanil) in combination with hyperbaric bupivacaine for Cesarean section, there is no consensus on the optimum dosage of fentanyl and sufentanil (3, 5-7, 22, 23).
Our study revealed that the use of fentanyl (25 mcg) and sufentanil (2.5 mcg) in combination with intrathecal bupivacaine was associated with more appropriate analgesia (312.5 minutes and 314 minutes, respectively) compared to 116 minutes by placebo during and after surgery. In a study by Braga et al. (21), duration of analgesia in the fentanyl group was 177 minutes and it was 210 minutes in sufentanil group, and this difference might be noted as a result of lower dose of administered bupivacaine (10 mg). However, Saraswat et al. (24) mentioned 322 and 409 minutes of analgesia by fentanyl and sufentanil groups, respectively. However, these higher durations might be attributed to higher dose of bupivacaine (15 mg) and sufentanil (10 mcg). Nevertheless, in both mentioned articles, adding fentanyl and sufentanil was associated with analgesic prolongations, which was consistent with our results.
In the present study, the peak of sensory block was noted at T4. Also, Neeta et al. (25) mentioned T4 and Motiani et al. (26) mentioned T6. In another study, peak sensory block was noted at T11 for sufentanil and at L1 by fentanyl (27). They used 4 mg of bupivacaine, which could justify a lower level of blocks in their study. Also, Karbasy et al. found that the height of block could be influenced by patients’ addiction, which might be a result of tolerance to opioids (28).
In our study, duration of sensory block in both S and F groups were longer than P group, and S group had longer duration than the F group, which was similar to previous investigations (24, 25). Motiani et al. (26) and Khara et al. (29) indicated longer duration of sensory block and analgesia in sufentanil group compared with fentanyl and placebo groups. However, Neeta et al. (25) reported longer blocking effect by fentanyl compared with sufentanil. However, Kim et al. (27) found no statistically significant difference between the 2 groups in returning sensory block, which was inconsistent with our results.
In the current study, the duration of motor block in S and F groups was longer than P group (320 and 291minutes versus 223 minutes). Braga et al. (21) and Khara et al. (29) mentioned longer duration of motor block in the sufentanil and fentanyl groups compared with the placebo group, which was similar to our study. In the study by Li et al. (30), results showed that no significant difference was obtained between groups in duration of sensory and motor block by administering equipotent dosages of fentanyl and sufentanil.
In our study, a significant reduction was obtained in mean arterial pressure and heart rate immediately after spinal analgesic injection in all 3 groups. Although administering fentanyl reduced blood pressure more than other drugs immediately after spinal analgesia, there was no significant statistical difference. The decreased blood pressure after intrathecal injection might be a result of reduced activity of the sympathetic afferent. Neeta et al. (25) as well as Kim et al. (27) mentioned no significant difference among groups in hemodynamic parameters, which was consistent with our study, and they further indicated a maintained intraoperative hemodynamic stability of these drugs.
With respect to complications, itching is the predefined complication of administering intrathecal opioid with the prevalence of 0% to 100%, which is related to the administered dose although the mechanism of itching is unknown yet. In our study, the frequency of itching in F group was higher than S and P groups (P = 0.003). Motiani et al. (26) and Dourado et al. (31) mentioned higher frequency of itching in sufentanil group than other groups. Although Braga et al. (21) mentioned no significant difference in the incidence of itching between the 2 groups as well, itching was much more noted in sufentanil group, and this result was inconsistent with ours. This difference in the results might be noted as a result of lower dosage of administered sufentanil in our study (2.5 mcg) compared to others (5 - 7.5 mcg).
The incidence of nausea and vomiting following intrathecal opioid injection is almost 30%. In our study, no significant difference was found among groups, which was similar to Motiani et al. (26). However, in a study by Lee et al. (32), no significant incidence of nausea and vomiting was found after administering the intrathecal opioid. Although nausea and vomiting during Cesarean section might be related to the manipulation in uterus and peritoneum, it was expressed that administering intrathecal opioid might have protective effect against nausea and vomiting (33, 34). These studies indicated that antiemetic drugs might be needed only when intrathecal local anesthetics have been administered and this might state the protective effects of lipophilic opioids on complications such as nausea and vomiting.
In this study, a higher frequency of shivering was seen in P group than other groups (P = 0.036), which was similar to a previous investigation (29). Onk et al. (34) and Faiz et al. (35) mentioned that using fentanyl or magnesium sulfate as an adjuant to spinal bupivacaine could prevent postsurgical shivering. Therefore, it seems that other factors such as operating room temperature, intravenous fluids etc. might affect it.
Sedation is a direct effect of opioids that may be desirable with no interference in mother- baby relationship. In this study, although in all groups most of patients had the sedation score of 2, there was a statistically significant difference among groups (P = 0.019). In the study by Lee et al. (32), a large number of patients, who received bupivacaine alone, had been fully awake and anxious, but a light sedation with easy arousal was noted in patients receiving opioids in combination with intrathecal bupivacaine, and these results were consistent with our study and supported the use of intrathecal opioid in combination with local anesthetic. Also, there was no significant difference between 3 groups in the first and 5th minutes Apgar score, which was consistent with the results mentioned by other studies (7, 36). Furthermore, Karbasi et al. found that intravenous fentanyl injection before anesthetic induction indicated no change in neonatal Apgar (37). Therefore, adding intrathecal opioid did not produce significant fetal depression.
Although investigators explained the safety of drugs for participants, some women were not willing to participate, and it prolonged the duration for sampling, which can be mentioned as a limitation of this study. Also, in this study, only one dosage of fentanyl and sufentanil was used for neuroaxial blocking, therefore, it is recommended to use and compare other intrathecal dosages of opioids and other nonopioid drugs in combination with intrathecal local anesthetics for further investigations to choose the most appropriate combination or the dosage for neuroaxial blocking in Cesarean section.
5.1. Conclusion
According to the results, adding 25 mcg fentanyl and 2.5 mcg sufentanil to intrathecal bupivacaine was associated with increased sensory block, motor block, duration of analgesia, and hemodynamic stability, with no major complication. Considering that the use of intrathecal fentanyl had a similar duration of analgesia like sufentanil, but it had a faster return of motor block and consequent ambulation, it seems that fentanyl is a preferred opioid for Cesarean section compared to sufentanil.