1. Background
Regional anesthesia is a safe and inexpensive technique which is widely used in cesarean section. It reduces the risk of airway complications and avoids hemodynamic changes associated with laryngoscopy and intubation (1, 2). Recently, application of intrathecal adjuvants has gained popularity with the aim of prolonging the duration of block, better success rate and patient satisfaction (1-4). Opioids such as sufentanil are commonly used as additives to local anesthetics to prolong the duration and intensify the effects of subarachnoid block (2). However significant side effects of opioids such as pruritus, urinary retention, respiratory depression, hemodynamic instability, occasionally severe nausea and vomiting may limit their use (1-3).
It has been shown that the duration of postoperative analgesia was prolonged when magnesium is given as an adjunct for peripheral nerve blocks (5). A few clinical trials have examined the effect of adding intrathecal MgSO4 to anesthetic agents such as bupivacaine (6-8), yet the mechanisms of action are not clear. Anti-nociceptive effects of Mg are probably due to regulation of calcium influx into the cell and antagonisation of N-methyl D-aspartate (NMDA) receptors. In the human body, calcium channel blockers can increase the analgesic effects of opioids. The analgesic effect of magnesium is due to its effect on NMDA receptors. N-methyl D-aspartate receptor has negative modulatory sites for agents such as magnesium. Furthermore, it is coupled with ion channels such as K+ and Ca2+. Magnesium causes a voltage-dependent block on NMDA receptors (6-10).
2. Objectives
The present study was designed to examine whether addition of intrathecal magnesium sulfate would enhance the analgesic efficacy of intrathecal bupivacaine in patients undergoing cesarean section.
3. Patients and Methods
Eighty patients with American Society of Anesthesiologist (ASA) class I-II, between 18 and 35 years old, scheduled for cesarean section under spinal anesthesia, were included in this prospective, randomized, double-blind clinical trial. After the ethic’s committee approval, written informed consent was obtained from all patients preoperatively. Patients with a history of long-term steroid therapy, uncontrolled hypertension, neurologic or psychological disorders, body weight > 100 kg or height < 150 cm, spinal column surgery, chronic pain, opium addiction or consuming drugs that modify pain perception were excluded from the study.
After IV line preparation, 10 cc/kg lactated ringers solution was infused to all patients. They received no premedication and electrocardiogram (ECG). Peripheral oxygen saturation (SPO2), and non-invasive arterial blood pressure (NIBP) were monitored upon arrival into the operating room and were recorded every five minutes until the end of the surgery and vital signs were registered every 15 minutes in the Post Anesthesia Care Unit (PACU). Patients were randomized by a computer-generated random number table into two groups of 40 patients:
Group I: Intrathecal administration of 10 mg of hyperbaric bupivacaine (Mylan, 20 mg/4 cc) and 0.5 mL of normal saline.
Group II: Intrathecal administration of 10 mg of hyperbaric bupivacaine and 50 mg of magnesium sulfate (Samen Daru, Iran).
Spinal anesthesia was given in the sitting position at the L4-L5 level through a midline approach using a 25-gauge Quincke spinal needle. After aspiration of 0.5 mL of cerebrospinal fluid, the local anesthetic solution was administered over 10-15 seconds. The patients were immediately returned to the supine position with left uterine displacement. To facilitate the double-blinding method, the medication was prepared and injected by an anesthesiologist who was not involved in the study. Thus, the patients and the observer were blinded to the study groups.
The sensory block level was assessed by a pin-prick test using a short bevel needle bilaterally along the mid-axillary line. The sensory block level was controlled every 30 seconds for 15 minutes, and then it was evaluated every five minutes until the end of the surgery. The onset was defined as the time between injection of drugs into the intrathecal space and the peak of sensory and motor block (highest dermatome level). The motor blockade was evaluated by the modified Bromage scale (0: patient can move their hip, knee and ankle; I: patient can move their knee and ankle; II: patient can move their ankle; III: patient can’t move their hip, knee and ankle) (11). The time of reaching to Bromage III is considered as the onset of motor blockade. Complete motor block recovery was assumed when the modified Bromage score was 0.
Time for regression of four sensory blocks was recorded. Pain assessment was done using the visual analog pain scale (VAS) with scores ranging between 0 and 10 (0 = no pain, 10 = most severe pain) every one hour. If the postoperative VAS was higher than four, the patient was treated by meperidine 20 mg IV. The interval between intrathecal drug injection and meperidine injection was considered as the duration of analgesia. Patients were observed for possible neurologic deficits at the time of discharge from the hospital. Hypotension was defined as a systolic blood pressure decrease of more than 30% from baseline. Hypotension was treated promptly by increasing uterine displacement and fluid administration. If hypotension persisted despite these measures, 5 mg of ephedrine was injected.
Bradycardia (heart rate < 50 beats/minute) was treated by IV atropine 0.5 mg. Decreased oxygen saturation under 92% or respiratory rate less than 10 times/minute was considered as respiratory depression. Sample size calculation was based on a previous study. With a significance level of 95%, power of study 80%, α error of 0.05 and β error of 0.2, to show a 20% difference in the duration of analgesia, at least 35 patients per group were needed (12). We took 40 patients per group for our study to compensate for any drop outs. Onset time, four sensory block regression, and duration of analgesia were analyzed by the t-test. For categorical covariates (sex, nausea/vomiting, hypotension, bradycardia), the comparison was done using a chi-square test or Fisher’s exact test. The significance level was defined as P < 0.05. Data were expressed as mean ± SD.
4. Results
All patients completed the study, with 40 patients in the magnesium sulfate group (case group) and 40 in the control group. The patients’ characteristics including age and weight were similar between the two groups (Table 1). The onset of sensory blockade in the bupivacaine group was more rapid than the magnesium sulfate group (P = 0.01), yet the onset of motor blockade was not different in both groups (P = 0.56) (Table 2). The duration of motor blockade was not significantly different between the two groups. Time for regression of four sensory blocks was similar (P = 0.19). Duration of analgesia was longer in the magnesium sulfate group yet this difference was not significant (P = 0.07) (Table 2).
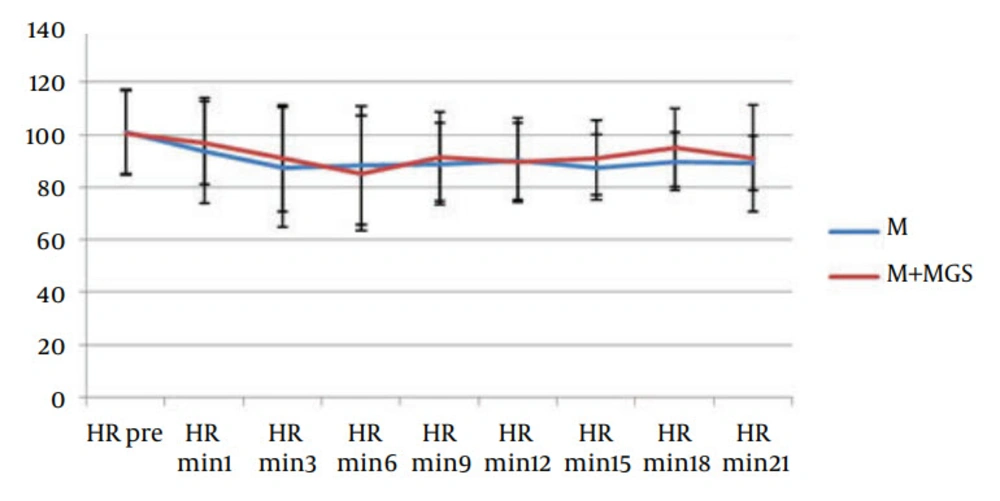
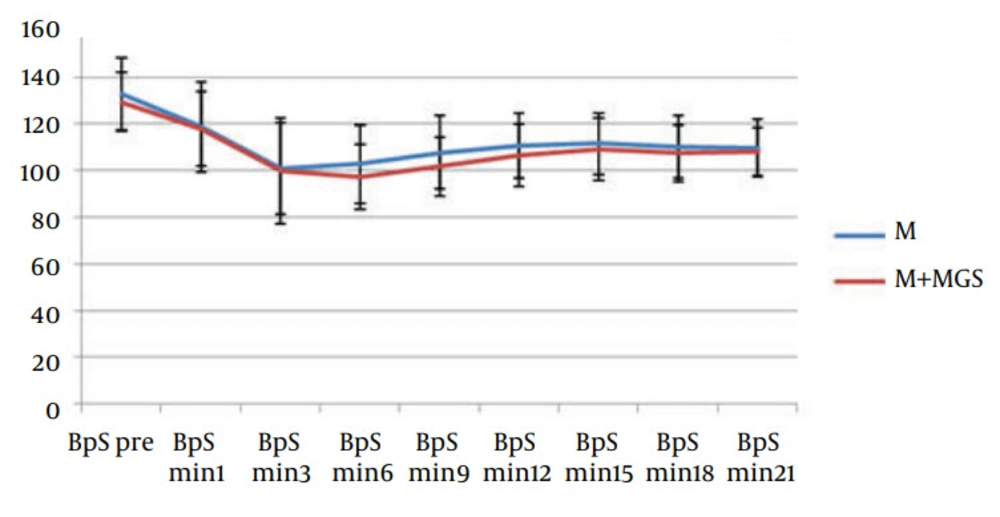
Hemodynamic parameters such as heart rate and systolic blood pressure were similar in both groups (Figures 1 and 2). Three patients in the magnesium sulfate group and one patient in the control group experienced nausea and vomiting. Postoperative follow-up revealed uneventful recovery in all patients. No neurologic deficit was observed in any of the patients. Apgar score of babies (at one, five and ten minutes) was similar in both groups.
| Variable | Control Group | MgSo4 Group | P Value |
|---|---|---|---|
| Age, y | 27.48 ± 4.49 | 26.88 ± 4.44 | 0.56 |
| Weight, kg | 78.13 ± 10.89 | 83.10 ± 11.91 | 0.08 |
a Data are presented as mean ± SD.
| Variable | Control Group | MgSo4 Group | P Value |
|---|---|---|---|
| Onset time of sensory block, min | 5.65 ± 0.92 | 6.60 ± 1.12 | 0.01 |
| Onset time of motor block, min | 8.57 ± 5.83 | 7.83 ± 5.67 | 0.56 |
| Duration of 4 dermatome block regressions, min | 61.33 ± 10.16 | 64.28 ± 10.07 | 0.19 |
| Duration of motor block, min | 56.75 ± 32.35 | 61.68 ± 39.37 | 0.54 |
| Duration of analgesia, min | 137.12 ± 9.95 | 160.87 ± 8.37 | 0.07 |
a Data are presented as mean ± SD.
5. Discussion
The main finding of this study was that in patients undergoing caesarean section under hyperbaric bupivacaine spinal anesthesia, the addition of 50 mg magnesium sulfate led to a significant delay in the onset of sensory blockade. Indeed, there was no significant difference in the duration of post-operative analgesia and characteristics of motor blockade in the two groups. The study showed that systemic administration of magnesium is associated with less analgesic requirement and less discomfort during the postoperative period (9, 13). Several experiments demonstrated analgesic effects of magnesium sulfate in neuraxial and peripheral block (5-7). It was observed that addition of magnesium sulfate to bupivacaine and fentanyl prolonged the period of anesthesia without additional side-effects (11). In some studies, it was observed that the visual analog scores, first analgesic requirement and total morphine requirement were significantly lower after intrathecal injection of magnesium sulfate (12, 14). In patients undergoing caesarean section under spinal anesthesia, the addition of intrathecal magnesium sulfate (100 mg) to morphine improved the quality and the duration of postoperative analgesia without increasing the incidence of adverse effects (15). Intrathecal magnesium was used for labor analgesia at the dose of 50 mg in addition to fentanyl; a significant prolongation in the duration of analgesia in the magnesium-fentanyl group compared with the fentanyl group was observed (16). Jabalameli and colleagues found that the duration of sensory and motor blockade was significantly prolonged by 75 mg or 100 mg magnesium sulfate compared with 50 mg magnesium sulfate (17). Unlugenc et al. showed that the addition of magnesium sulfate to bupivacaine did not shorten the onset time of sensory blockade or prolong the duration of spinal anesthesia (18). These differences may be due to the dose of intrathecal bupivacaine and magnesium or different surgical procedures.
In our study, we found that the onset of sensory blockade was delayed with the addition of intrathecal magnesium. This finding is similar to a previous study in which it was shown that the addition of intrathecal magnesium sulfate to bupivacaine and fentanyl anesthesia delayed the onset of the sensory and motor blockade (16). Ozalevli et al. observed a similar delay in onset of spinal anesthesia when adding intrathecal magnesium sulfate to fentanyl and isobaric bupivacaine (11). They suggested that the differences in pH and baricity of the solution containing magnesium sulfate contributed to the delayed onset (12, 16).
The optimal dose of intrathecal magnesium has not been reported previously. The dose of magnesium used in this study was based on data from the study of Buvanendran et al. where 50 mg of spinal magnesium sulfate potentiated fentanyl antinociception (16). We suggest further researches in the future, with different magnesium dosages and more patients, to determine the safest route and dosage. This study shows that the addition of intrathecal magnesium sulfate to bupivacaine is not desirable in patients undergoing cesarean section due to the delay in the onset of sensory blockade and the lack of significant effects of magnesium on post-operative pain.