1. Background
Peripheral nerve stimulation (PNS) refers to the placement of a lead near the anatomic location of a nerve to directly stimulate it (1). This technique has been used to the cranial nerves for treatment of chronic intractable headache of various types (2-4), including migraine (5-7), cluster headache (8, 9), occipital neuralgia (10, 11) cervicogenic headache (12) and supraorbital neuralgia (13). Promising outcomes have been reported, with efficacy rates in pain reduction and functional improvement ranging from 40% to100% (2-4, 8, 14, 15). While this positive response is encouraging, complications of the therapy, that include acute and late-onset infections, lead migration and skin erosion, have been of concern (3, 4, 6-8, 14-16). These complications significantly impact the clinical application of this treatment modality, and could impede a more widespread use of it in the future.
2. Objectives
Here we report the results of peripheral nerve stimulator implantation performed on 24 patients with various chronic headaches at our center over a period of 9 years. We describe the complications of the procedure and their prevention with a modified surgical technique.
3. Patients and Methods
This was a retrospective chart review, conducted at a large academic medical center in an urban setting and at a private practice pain center. The study was approved by the Thomas Jefferson University institutional review board. All patients were initially evaluated for headaches. The study period was from August 2006 to January 2015.
We searched our database for patients] with chronic refractory headaches, who had undergone PNS for this indication. These patients had failed multiple conservative therapies, including medications, psychotherapy and physical therapy. They were referred by headache specialists to consider PNS therapy.
All patients were thoroughly evaluated at our center by one of the authors (LZ). They also underwent a psychological evaluation before a PNS trial was considered. The decision as to which scalp nerve to target for stimulation was based on the patient’s clinical presentation and headache diagnosis. For patients who experienced > 50% headache reduction during the 7 day trial, implantation of a permanent PNS was approved (of the total patients who underwent the trial, all but one met the above criterion, received a permanent PNS implantation, and participated in the study). Implantation areas included the occipital nerve, supraorbital nerve and auriculotemporal nerve. Implantations were done uni or bilaterally.
Clinical outcomes, including Total Pain Index (TPI) (17), patient satisfaction and complication rates, were documented before and after surgery. TPI (total pain index): an integrated measure of the intensity and duration of headache attacks over a two week period defined by the Formula 1:

where D1, number of hours with headache that is slight, does not limit normal activity; D2, number of hours with headache that is moderate, limiting normal activity but does not confine patient to bed; D3, number of hours of headache that is severe, limiting all activities causing patient to be bedridden (17).
All patients were seen approximately 14 days after the procedure, then monthly for 6 months. Thereafter, they were followed every six months, either at the clinic or by a phone call, for 6 months to 9 years (average follow-up period: 4.9 years). Outcome evaluation was done by asking the patients about their pain intensity, as well as duration and frequency of headaches, before the procedure and at each follow-up interview. Patient’s satisfaction and treatment complications (if any) were also documented. Statistics:
We used student’s t-test to compare pain parameters before treatment and at follow up. The level of statistical significance was set at 0.01.
4. Results
We identified 24 patients (5 males, 19 females), who had undergone PNS for their chronic refractory headaches. Their mean age was 45.8 years (range: 22 - 66 yeas), and their average headache duration was 10.3 years (range: 2 - 25 years). Patients were diagnosed with chronic migraine (15/24), occipital neuralgia or cervicogenic headache (9/24).
Three of the 24 patients were not followed after their leads were removed. The remaining 21 patients were followed periodically as described above.
All 24 patients experienced significant headache relief for three months after permanent device implantation, with a decrease in pain intensity, duration and frequency (TPI). Mean TPI decreased significantly, from 516 ± 131 before the procedure to 74.8 ± 61.6 at the last follow up (P < 0.00001). Three patients had their stimulator removed; one due to skin erosion and infection at the silastic anchor site (as mentioned above), the second due to skin allergic reaction to the stimulation lead (18), and the third because of her religious belief. The remaining twenty-one patients have been followed since surgery. They have maintained the same degree of pain relief throughout the follow up period without significant complications.
Overall, the surgical outcomes were excellent in these 24 patients (Table 1). There were no acute post-operative infections. In the early three patients of this group, silastic anchors were used. One of them had lead migration one year after implantation, requiring revision. The same patient also developed scalp erosions on both sides at different times of treatment, requiring revision. After re-implantation with modified technique without silastic anchors, he has had no further complications, keeping his stimulation therapy. Another one of the early three patients developed skin erosion at the silastic anchor site two years after implantation, requiring lead removal. Due to these problems, the surgical technique was modified, with significant reduction in complication rate (Tables 1 and 2). After revision of the surgical technique, 21 consecutive patients were treated and evaluated. In this group, there were no major post-operative complications, such as skin erosions, infection or lead migrations. The self-rated treatment satisfaction was excellent in 13 of the 24 (54%) patients, very good or good in 10 (42%), and fair in 1 (4%) patient.
| No | Sex | Age, y | History of HA | HA Frequency | HA Duration | TPI | TPI Reduction (%) | Nerve(s) Stimulated | Self-Rated Outcome | F/U Duration | Comments (See Tab 2) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |||||||||
| 1a | F | 47 | 14 | daily | rarely | constant | 2 h | 574 | 140 | 75.6 | L. occi /supra/auri | good | 9 | |
| 2a | M | 48 | 5 | daily | 1 - 2/w | constant | 2 - 3 h | 390 | 88 | 77.4 | Bil. occi/supra/auri | good | 9 | 1 |
| 3a | M | 53 | 15 | daily | 1 - 2/w | constant | 2 - 3 h | 854 | 124 | 85.5 | Bil. occi | excellent | 2 | 2 |
| 4 | F | 54 | 15 | daily | none | constant | none | 364 | 0 | 100 | Bil. occi/supra | excellent | 5 | 3 |
| 5 | F | 46 | 6 | daily | rarely | constant | 2 - 3 h | 630 | 28 | 95 | Bil. occi /supra | good | 7 | 4 |
| 6 | F | 42 | 5 | daily | 2/w | constant | 2 - 3h | 420 | 48 | 88.6 | L. occi/supra | good | 7 | |
| 7 | F | 62 | 15 | daily | 1 - 2/w | constant | 3 - 4 h | 840 | 36 | 95.7 | Bil. occi | excellent | 7 | |
| 8 | F | 50 | 20 | 5/w | none | constant | none | 504 | 0 | 100 | Bil. occi | excellent | 7 | |
| 9 | M | 59 | 15 | daily | daily | 8 - 9 hours | 2 - 3 h | 364 | 140 | 61.5 | L. occi/supra | fair | 6 | |
| 10 | M | 66 | 20 | daily | none | 0.5 to 1 h | none | 336 | 0 | 100 | L. occi/supra | excellent | 6 | |
| 11 | F | 38 | 5 | daily | rarely | constant | 1 - 2 h | 350 | 32 | 90.9 | R. occi/supra | excellent | 6 | |
| 12 | F | 50 | 20 | daily | rarely | constant | 1 - 2 h | 364 | 32 | 91.2 | R. occi/supra | excellent | 6 | |
| 13 | F | 60 | 20 | daily | none | constant | none | 434 | 0 | 100 | L. occi | excellent | 6 | |
| 14 | F | 41 | 3 | daily | 1/w | constant | 1-2 h | 616 | 10 | 98.4 | Bil. cci/supra/auri | very good | 6 | |
| 15 | F | 51 | 5 | daily | 3/w | constant | 1 - 2 h | 504 | 16 | 96.8 | Bil. occi | excellent | 6 | |
| 16 | M | 31 | 3 | daily | none | 8 - 12 h | none | 390 | 0 | 100 | L. occi/supra | excellent | 5 | |
| 17 | F | 33 | 2 | daily | rarely | constant | 0.5 - 1 h | 476 | 4 | 99.2 | R. occi/supra | excellent | 4 | |
| 18 | F | 23 | 3 | daily | 3 - 5/w | constant | 2 - 3 h | 532 | 124 | 76.7 | Bil. supra | good | 4 | |
| 19 | F | 48 | 5 | daily | 2 - 3/w | constant | 2 - 3 h | 530 | 120 | 77.3 | R. occi/auri | excellent | 4 | |
| 20 | F | 37 | 3.5 | daily | 3/w | constant | 1 - 2 h | 420 | 96 | 77.1 | R. occi/supra | good | 2.5 | |
| 21 | F | 24 | 5 | daily | daily | constant | 12 h | 840 | 336 | 60 | Bil. occi/supra | good | 1.5 | |
| 22 | F | 22 | 13 | daily | daily | constant | 14 h | 868 | 280 | 68 | Bil. occi/supra | good | 1.5 | |
| 23 | F | 56 | 25 | daily | rarely | constant | 1 - 2 h | 360 | 60 | 83.3 | Bil. occi | excellent | 0.3 | 5 |
| 24 | F | 58 | 5 | daily | rarely | constant | 2 - 3 h | 435 | 80 | 81.6 | Bil. occi | good | 0.3 | 6 |
Abbreviations: Freq, frequency; HA, headache; Dur, duration; Bil, bilateral; L, left; R, right; h, hour; F/U, follow-up; occi, occipital nerve; supra, supraorbital nerve; auri, auriculotemporal nerve.
aPatients number 1 - 3 were the early cases with silastic anchors. The other patients (numbers 4 - 24) were treated using the revised surgical technique.
| No. | Complications | Years after the implant | Management | Outcome |
|---|---|---|---|---|
| 1 | Excellent. Pt remained with leads | |||
| One supraorbital lead migration | 1 year | Lead revision | ||
| Infection over one side of plastic anchor due to skin erosion | 1.5 years | Local debridement & lead revision | ||
| Infection at the other side due to a similar cause | 2 years | Lead revision | ||
| 2 | Infection over the plastic anchor due to skin erosion | 2 years | Device removal | |
| 3 | Pain at the battery sites (subclavicular) | 3 years | Batteries relocated from the infraclavicular fossa to the buttocks | Excellent. Pt remained with leads |
| 4 | Infection at neck over the anchor knot | 4 years | Debridement | Excellent. Pt remained with leads |
| 5 | Skin allergic reaction to the lead component | 3 months | Failed conservative therapy & device removal (18). | |
| 6 | The patient reported significant headache reduction after implantation. However, three months later she requested device removal due to her religious beliefs. | 3 months | Device removal |
5. Discussion
We found that PNS of various nerves in the cranial area was effective and well tolerated in our patients with previously refractory chronic headaches. The mechanism of the analgesic effect of peripheral neurostimulation may be related to activation of large fiber sensory afferents, that in turn inhibit nociceptive fiber activity (15).
In a previous study, Schwedt et al. (4) reported on a mean subjective pain improvement of 52 after occipital nerve stimulator implantation in 15 patients with refractory headaches of various etiologies, however, most patients required lead revision within 1 year. Our efficacy results are comparable to those reported in that study, with a lower complication rate, possibly due to our technique modifications.
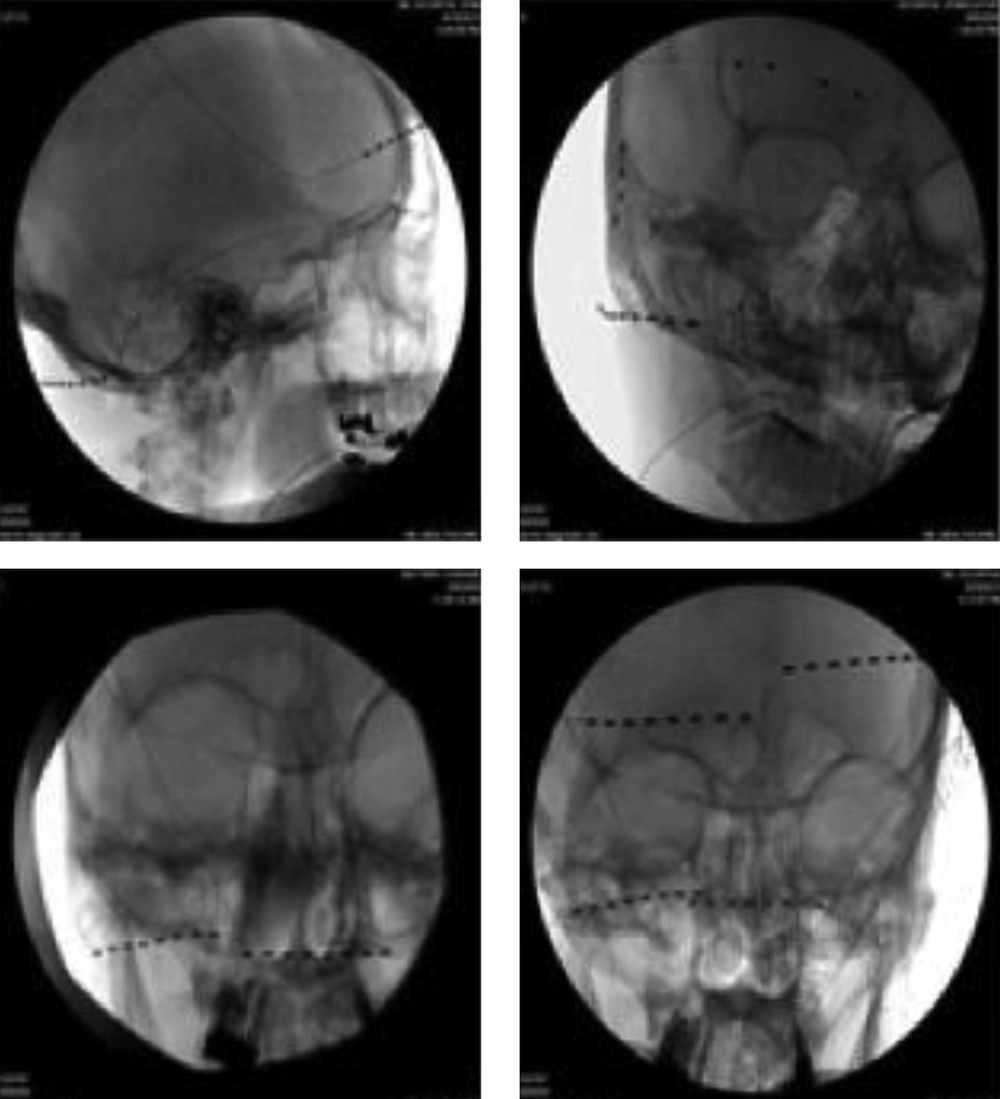
5.1. Selecting Scalp Nerve(s) for PNS (Figure 1)
In order to achieve better headache relief, selecting cranial nerves for PNS therapy is important, which is based on the patient’s clinical presentation and headache location. The historical database for PNS therapies suggests that concordant neurostimulation may provide better headache relief than a non-concordant one (15, 19). If the patient presents with one-sided occipital headache, a single 8-electrode lead with regular contact and contact spacing lead is applied to cover all three occipital nerves (greater, lesser, and third ) unilaterally. If the patient presents with headache at the occipital and frontal regions, two 8-electrode leads are used, one for the occipital nerves, and the other for supraorbital and supratrochlear nerves, respectively (Figure 1 top left). If the patient presents with headache at the occipital, frontal and temporal regions, an 8-electrode lead is used for the occipital nerves, one 4-electrode with wide electrode contact spacing lead is used to cover the supraorbital and supratrochlear nerves, and one 4-electrode with small contact spacing lead to the auriculotemporal nerve (Figure 1 top right). Two 4-electrode leads are converted to the 8-contact extension lead; the latter with the other 8-electrode lead is connected to the same implantable pulse generator (IPG). If the patient presents with bilateral occipital headache, two 8-electrode leads are used for both sides of the occipital nerves (Figure 1 bottom left). If the headache is at the occipital and frontal regions bilaterally, based on the clinical presentation, multiple leads are used (Figure 1 bottom right). We found that leads from a unilateral side (occipital and supraorbital) connected to the same IPG provide better pain relief compared with two occipital leads connected to one IPG and the other two supraorbital leads connected to a different IPG. We believe that the frontal and occipital leads from the same IPG can produce a broad paresthesia (cross talk electro-magnetic field) between these two leads, which gives better pain relief. The current 32-contact devices allow the use of 4 independent 8-contact electrodes with a single IPG.
5.2. Surgical Technique of PNS
Common complications of PNS include infection, skin erosion and lead migration (3, 4, 6-8, 14, 16). After encountering these complications in our early cases, we have modified our surgical techniques. In our twenty-one patients treated with modified technique, none developed lead migration, skin erosion and scalp infection.
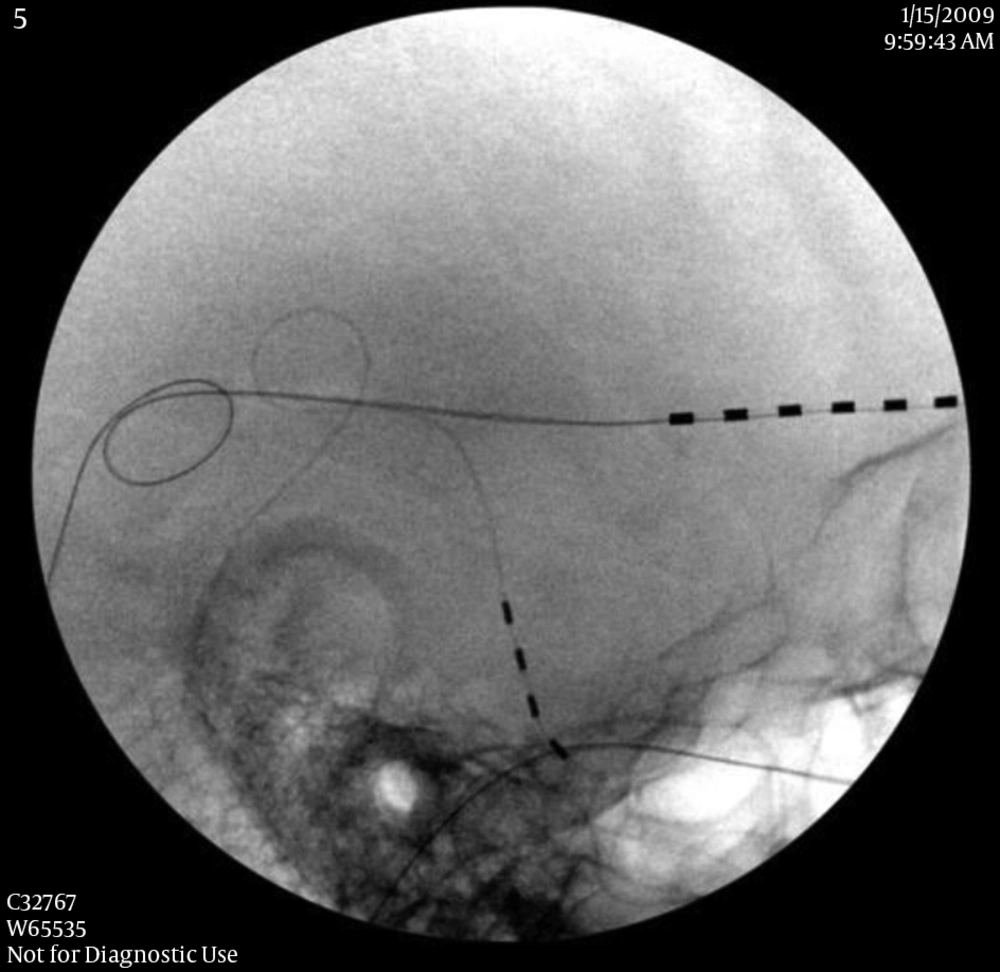
The followings are the key points of permanent implantation of PNS. The skin need to be appropriately prepared with haircut at least 3 inches from the incision sites, and marked for the incision and needle entrance. When selecting the incision and needle entrance sites, the cosmetic and soft tissue factors (to cover the lead and anchors) should be considered. After the incision is made, minimally invasive hemostasis techniques are used to prevent skin and subcutaneous tissue damaged, because the damage can cause a difficulty to close the incision and result in infection. After exploring the fascia and making a subcutaneous space between scalp and fascia, two 2 - 0 non-absorbable sutures are sutured and tightened to the galea aponeurotica (fascia) as anchors with 0.5 to 0.8 inch apart as a tension releasing loop (Figure 2). A pre-bent introducer is inserted through the incision toward the target nerves. After the introducer reaches the appropriate position, the PNS lead without a stylet is inserted under fluoroscopic guidance. After removal of the introducer, the PNS lead is directly tightened to the galea aponeurotica as an anchor. In order to prevent lead migration, it is very important to tighten the suture with a standard technique. We found that directly tightening the PNS lead will not cause any damage to it (in our group for 9 years of follow-up). It may require multiple tension releasing loops for the PNS leads relaying to the IPG. When tunneling the introducer from the temple to the occipital region, the needle should be away from the superior part of the external ear in order to prevent glasses earpieces irritating the lead or causing skin breakdown. Each layer of scalp tissue should be closed properly. If the tip of the PNS lead is found penetrating the skin or tilted towards the skin, the PNS tip can be buried deeply using a retrograde technique (20). After surgery, the patient should avoid rapid or extreme flexion and extension of the spine for several weeks, to allow fibrosis and firm attachment of the leads to the surrounding tissue.
5.3. Summary
Our results support the use of PNS in some patients with refractory chronic headaches, and add to the existing data on this treatment modality. Selecting the appropriate cranial nerve(s) for PNS therapy is essential to maximize the headache relief. An appropriate and meticulous surgical technique is crucial to prevent procedure-related complications; most commonly lead migration, skin erosion and infections.