1. Background
Traumatic brain injury (TBI) is one of the serious causes of mortality and disability worldwide, and it is estimated that annually about 1.5 million people die and millions of them need to emergency care because of TBI. The mortality rate after TBI depends on the intensity of injury and mechanism underlying the trauma although adverse outcomes may reach 120 percent (1, 2). Wide cognitive and physical disability and a high TBI-related mortality rate interested the researchers to explore the ways of diagnosis and prognosis of this problem in order to proceed for better prevention strategies (3, 4). Today, determination of mortality and complications among patients admitted to intensive care units (ICUs) is one of the research priorities (5). Considering the cost and limitations of beds in ICU, it is very critical to determine the status of a patient in order to accept the most urgent patients (6, 7). Therefore, the personnel should select the patients who need urgent critical care on time, through appropriate tools (6, 7). Categorization of the severity of disease helps to judge about the treatment process, according to their demands and hospital facilities (8, 9). Considering critical situation of TBI patients for appropriate treatment (10, 11), there are several existing tools to estimate the hospital’s mortality rate of these patients in ICU (12). Glasgow coma scale (GCS) is the most common clinical tool for primary determination of TBI (13). Several researches have shown the efficacy of GCS in prediction of mortality and morbidity (13-16). However, GCS is an appropriate tool to determine the severity of TBI, but still includes some limitations (13-18). Therefore, other tools also designed during time, for example, Wijdicks et al. (19) invented a tool named full outline of unresponsiveness (FOUR) in order to overcome on the limitations of GCS. This tool provides information about brainstem reflex follow-up eye and respiratory patterns, which is ignored in GCS. The FOUR measures different stages of brain herniation and locking syndrome. In addition, FOUR can assess patients in the critical condition because it does not need verbal ability (20, 21). The appropriate relation of FOUR scores and outcomes was surveyed in several studies (22). In the last two decades, several researchers suggested designing more efficient tools (23, 24). Acute physiology and chronic health evaluation (APACHE II) is one of the suggested tools, it has been used worldwide since 1985 as a physiological parameter (25). Some studies compared APACHE with GCS and other related tools (23-26). However, the result of some researchers suggested that APACHE II is not efficient in patient’s undergone neurosurgery (23-27), but comparing the three tools (APACHE II, APACHE III and GCS) showed similar outcome predictions (28). In another study, comparison of GCS and APACHE II in patients with head trauma revealed GCS is better than APACHE II in prediction of outcome of head trauma; however, APACHE II is better in predicting the outcomes of other traumas (25). Glasgow coma scale was also compared with FOUR and findings indicated same efficacy (22-29).
2. Objectives
The present study aimed to compare the prediction of mortality rate among patients with TBI admitted to ICU.
3. Patients and Methods
This study was a diagnostic study. The proposal of the study was approved in ethical committee of Mazandaran University of Medical Sciences and a consent form was signed by caregivers of the patients.
3.1. Sampling and Procedure
The study conducted on 80 patients with TBI who suffered from the impaired consciousness level in the ICU of Mazandaran University of Medical Sciences during 2012 - 2013. The sample size calculated based on MedCalcR, keeping power = 90, α-level as 0.05 and β-level as 0.10, the sample size was calculated (22). The samples were selected using the purposive sampling method with a checklist based on exclusion and inclusion criteria. The inclusion criteria included TBI, age between 16 and 65 years old (30, 31), and admitting to the hospital more than 24 hours. The exclusion criteria were addiction history, trauma surgery problems, coma (GCS < 7), and using sedative medicines before evaluation. The checklist included demographic information, type of injury, and consciousness level according to FOUR, GCS and APACHE II, etc. The patients were evaluated by the researcher through GCS, APACHE II and FOUR in first 24 hours in order to predict delayed and early mortality. Early and delayed mortality: early mortality means the patient death before 14 days and delayed mortality means the patient death 15 days after admitting to hospital.
3.2. Tools
a) Glasgow coma scale is a standard tool to determine severity of impairment in TBI, which is accepted by physician and neurologists worldwide. It is comprised three subscales i.e. verbal (5 items), ocular (4 items) and motor (6 items) ranged between 3 and 5 (13).
b) Full outline of unresponsiveness comprised four subscales i.e. ocular, motor, brain stem reflex and breathing pattern scored between 0 and 4 and the total score is between 0 and 16. Reliability and validity of FOUR have been proved in several studies (9-33).
c) Acute physiology and chronic health evaluation II assesses blood pressure, heart rate, temperature, respiratory rate, mean arterial pressure, oxygen pressure contribution, pH, sodium and potassium and serum creatinine, hematocrit, white blood cell count, and arterial blood and ranged between 1 and 7. Several studies reported appropriate validity and reliability of this tool (26-28).
3.3. Statistics
The data were analyzed through logistic regression with the 0.95 confidence level. In order to compare the three scales in the prediction of mortality, the ROCI curve was used to show cut-off point and area under the curve (AUC). P < 0.05 was considered statistically significant. Values are expressed as mean ± standard error.
4. Results
The mean age of the patients was 33.80 ± 1.60 and their age ranged from 16 to 60 years old. From a total of 80 patients, 16 cases (20%) were females and 64 (80%) were males. The admission causes were as follows: 20 cases with epidural hematoma, 10 cases with subdural, 18 cases with cerebral edema and 32 cases with brain hemorrhage (Table 1).
| Variable | No. (%) |
|---|---|
| Gender | |
| Male | 64 (80) |
| Female | 16 (20) |
| Occupation | |
| Unemployed | 16 (20.8) |
| Home keeper | 10 (13.2) |
| Retired | 5 (5.7) |
| Employed | 8 (9.4) |
| Others | 41 (50.9) |
| Education | |
| Illiterate | 8 (9.4) |
| Elementary | 16 (20.8) |
| High school | 32 (39.6) |
| Academic | 24 30.2 |
| Marital status | |
| Single | 47 (58.5) |
| Married | 31 (39.6) |
| Widow | 2 (19.6) |
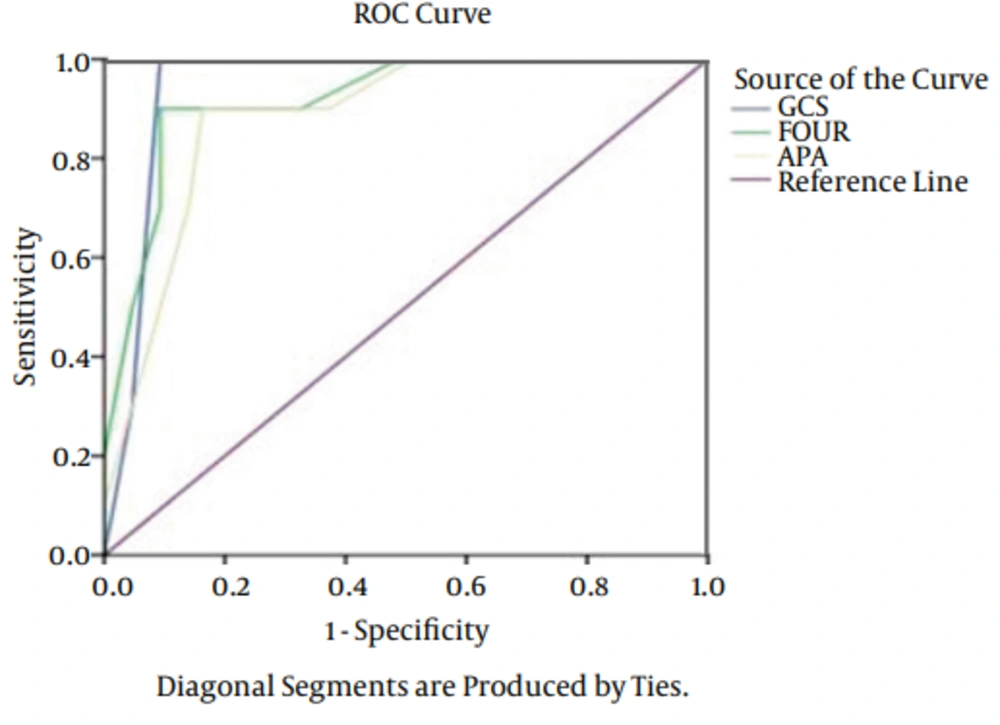
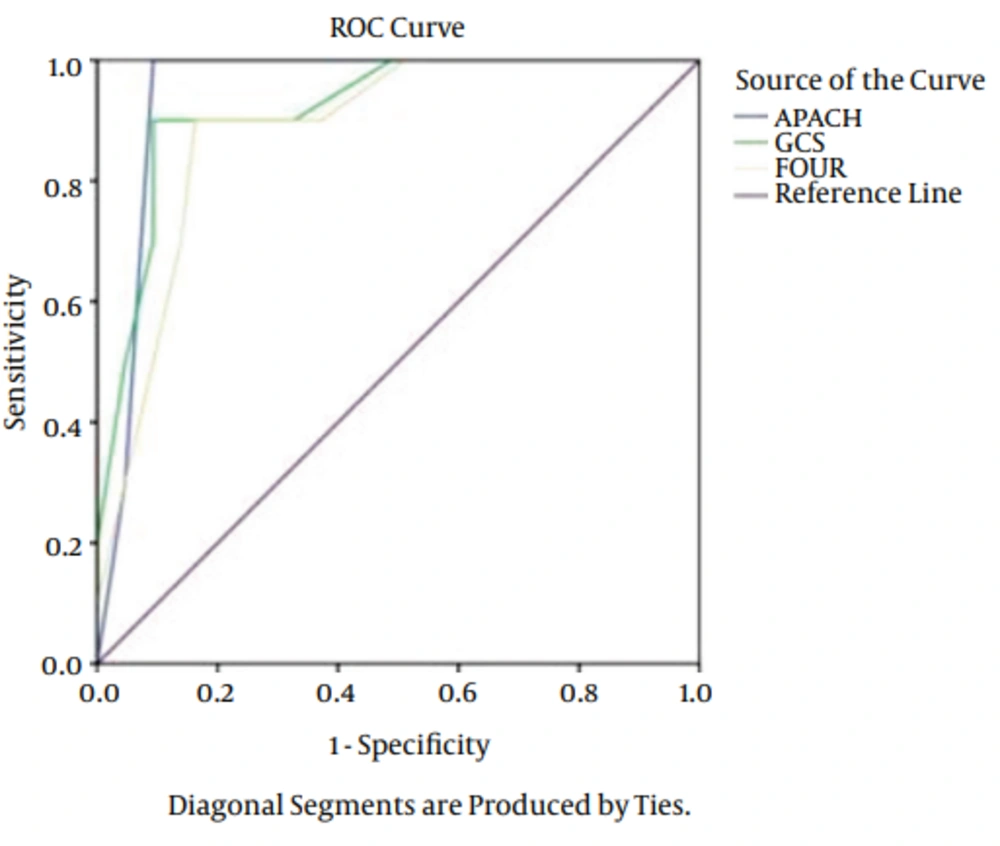
The types of injury were comprised: 42 cases of motorbike accidents, 25 cases of car accidents and 13 cases fell from a height. As it is obvious, the major cause of TBI was motorbike accident. The patients admitted because motorbike accidents were in the severe level compared to other cases. Of 80 patients, 15 (18.7%) patients expired, 11 (13.7%) of them expired before 14 days and 4 (5%) after 15 days. Results of logistic regression showed no significant relationship between age, gender and cause of admission with outcomes (P > 0.05). A significant relationship was found between outcomes of FOUR, APACHE II and GCS. Totally, the predictive power was appropriate in outcomes of APACHE II, GCS, and FOUR. The AUC of FOUR in early mortality was 0.92 (CI = 0.95. 0.81 - 0.97), GCS was 0.96 (CI = 0.95. 0.87 - 0.990) and APACHE II was 0.92 (CI = 0.95. 0.81 - 0.97). In delayed mortality the AUC was 0.94 (CI = 0.95. 0.74 - 0.97) for FOUR, 0.90 (CI = 0.95. 0.87 - 0.95) for GCS and 0.94 (CI = 0.95. 0.74 - 0.97) for APACHE II (Figures 1 and 2). The sensitivity of FOUR in the prediction of early mortality was 0.90 and 0.90 in cut off 4, APACHE II was 0.80 in cut off 14 and 0.75, and GCS was 0.92 and 0.100 in cut off 4. In terms of delayed mortality prediction, the AUCs were 0.90 and 0.80 in cut off 6 for FOUR, 0.88 and 0.75 in cut off 7 for GCS, 0.90 and 0.88 in cut off 17 for APACHE II. The values of cut off sensitivity and index with AUC presented in Tables 2 and 3.
| Score | Cut-Off Point | Specificity | Sensitivity | Youden Index | ROC Area |
|---|---|---|---|---|---|
| GCS | 7 | 0.88 | 0.64 | 0.75 | 0.90 ± 0.03 |
| FOUR | 6 | 0.90 | 0.73 | 0.80 | 0.89 ± 0.03 |
| APACHE II | 17 | 0.90 | 0.75 | 0.88 | 0.94 ± 0.02 |
Abbreviations: APACHE II, acute physiology and chronic health evaluation II; GCS, glasgow coma scale; FOUR, full outline of unresponsiveness.
| Score | Cut-Off Point | Specificity | Sensitivity | Youden Index | ROC Area |
|---|---|---|---|---|---|
| GCS | 4 | 0.92 | 0.100 | 0.84 | 0.96 ± 0.03 |
| FOUR | 4 | 0.90 | 0.90 | 0.80 | 0.92 ± 0.03 |
| APACHE II | 17 | 0.75 | 0.80 | 0.68 | 0.90 ± 0.02 |
5. Discussion
Subjective evaluations of clinical status of patients by individual clinicians may differ in terminology, and even this difference may appear in measurement of the severity of illness. Hence, several descriptive and prognostic evaluation scales have been developed during the past three decades. Objective evaluation of clinical status would facilitate comparison of methods, staff, clinical centers and studies. The need for such evaluation scales is particularly evident in coma patients (26). Therefore, this study compared GCS, FOUR and APACHE II in one study in the prediction of three tools among patients with TBI. The result showed that the majority of admitted patients were young males; this is not a surprising result as the main reason also was motorbike accident because the young males engage more frequently in risky behaviors compared to females and aged people. This result is similar to findings of other studies (22-35). In addition, results revealed that there was no relationship between age, gender, injury type and injury cause with outcomes, which is consistent with the result of a study conducted with Fakharian et al. (34). However, in a study done by Gan et al. (36) there was a significant relationship between outcome and injury mechanism and this can be due to the exclusion criteria of this study as we excluded patients above 65 years old. Furthermore, there was a significant relationship between GCS and FOUR scores with the outcomes of trauma. The prediction power was relatively similar in all three tools respectively; FOUR (AUC = 0.9), GCS (AUC = 0.96) and APACHE II (AUC = 0.90) in early mortality and FOUR (AUC = 0.89), GCS (AUC = 0.90) and APACHE II (AUC = 0.94) in delayed mortality. These findings are similar to the findings reported with Bastos et al. (37), Cho and Wang (28), Grmec and Gasparovic (26) although the mentioned studies do not included FOUR in their evaluations. In the present study, APACHE II showed better power prediction for delayed mortality and this result is explainable with characters of APACHE II which is evaluating physiologic factors, as several physical aspects are related to delayed mortality. This finding also is in accordance with the results of Cho and Wang (28). In a study conducted by Grmec and Gasparovic (26), GCS values determined before hospitalization of the patients and the APACHE II scores determined on the first day of hospitalization enabled them to compare descriptive and prognostic scales directly. They found that the prehospital GCS assessment was as good a predictor of mortality as was the APACHE II score, as measured in the hospital. On the other hand, Eken et al. (38) reported that in patients presenting with an altered level of consciousness, head trauma, or any neurological complaints on an emergency department, the FOUR-EM had a similar predictive value for unfavorable outcomes as the total FOUR score and the GCS. Their finding is in line with the work of Gill et al. (39) showing that the three individual GCS components alone performed similar to the total GCS score for the prediction of 4 clinically relevant TBI outcomes. Romera (40) also demonstrated that the GCS score correlates with APACHE II score in patients who have suffered a cerebrovascular accident. It was concluded that GCS is superior because of its simplicity and rapidity for prediction of mortality in patients with cerebroventricular accident. The authors of the present study also acknowledge the simplicity, rapidity and popularity of GCS regardless of the etiology of coma; however, when the situation of a patient is very sensitive and the clinician needs more information, APACHE II is more efficient in the prediction of delayed mortality. Also, FOUR has several advantages such as balance of items, diagnosis of locking syndrome, and evaluation of patients with intubation. The findings of this study revealed the relatively same efficacy in the three tools in the prediction of early mortality. However, APACHE II was more efficient in the prediction of delayed mortality, FOUR has several advantages such as balance of items, diagnosis of locking syndrome, and evaluation of patients with intubation and GCS was a simple and short tool to use. Therefore, the authors suggested that physician and nurse investigate patients’ conditions with each tool considering the advantage and disadvantage of tools in the routine examination of TBI patients in the same time. The complementary information of FOUR and APACHE II beside GCS can give some critical information and insights, especially about complicated cases. Glasgow coma scale and FOUR are better to use in the first 24 hours and APACHE II is more efficient in the next stages to predict the delayed mortality.