1. Background
Insufficient relief of postoperative pain is accompanied by complications such as long recovery and hospitalization, an increase of hospital costs, and reduction of patient’s satisfaction score (1). Effective management of postoperative pain is currently a part of the surgical process and not only reduces the patient suffering but also reduces mortality and promotes rapid hospital discharge, improves patient’s quality of life, and reduces hospital costs (1-4). Patient-controlled intravenous analgesia (PCIA) is an effective method for pain control, which has entered the literature since 1990 (5). Since the introduction of an analgesic pump, the usefulness of this method has been evaluated in contrast to the conventional nurse controlled analgesia (NCA) method. Advantages of the PCIA method include reducing the waiting time of the patient from pain sensation to pain relief, the workload of personnel and nurses and the probability of medical errors, and recording the injected dose more precisely (6). On the other hand, studies have shown that PCA has a greater reduction in pain intensity and better efficacy in controlling pain than other analgesics. However, these systems have some disadvantages such as device cost or lack of bolus injection in some models, which can lead to periods of silence and pain intensity in the patients (7).
Owing to some side effects such as drug overdose, delayed analgesia onset and ineffective postoperative pain control, some limitations occur; thus adding adjuvants, especially to opioid pumps has been proposed to decrease these problems (5). Recent studies have shown the beneficial role of adding nitroglycerin to other analgesic drugs such as lidocaine to upgrade the motor and sensory blockade, tourniquet pain, and postoperative analgesia. Even though the results are controversial due to different drug doses (8-12). Studies have also shown that transdermal nitroglycerin is effective in improving the analgesic effect of opium with nitric oxide release mechanism. It means that opium produces nitric oxide, in which increases the intracellular cGMP concentration to modulate the transfer of pain in the central and peripheral nervous system (13-16) and it can be used as an additive and may synergistically affect opioid by these mechanisms.
2. Objectives
Since there has been no previous study on the postoperative analgesic effect of intravenous nitroglycerine, the aim of this study was to evaluate the effects of nitroglycerin addition on the infusion of intravenous analgesia pump in patients with lower limb orthopedic surgery.
3. Methods
This study is a double-blind clinical trial conducted on 75 patients with tibia, fibula, and distal femur fracture. Qualified patients who underwent orthopedic surgeries were randomly divided into three groups using random number table. The inclusion criteria were patients aged 18 - 50 years, ASA I and II, blood loss less than available blood loss during surgery and surgery duration less than two hours. The method of anesthesia for these patients was spinal anesthesia with 15 mg bupivacaine 0.5%, without the use of any intravenous opioid for sedation. Exclusion criteria were lack of patient dissatisfaction to continue the study, sensitivity to any of the studied drugs, seizure, addiction to alcoholism, benzodiazepine and opium, heart failure, arrhythmia and valvular heart failure, Hypertension, and any history of neurological defects.
In this study after completing descriptions for the patients, written informed consent was obtained before the entrance to the operating room. All patients were randomly assigned to three groups of 25 patients using an intravenous analgesia pump (accufuser CTx, South Korea) at an infusion rate of 6 ml/h as follows:
Group A: 25 patients with fentanyl 10 mc/kg + 10 mL distilled water + 100 mL normal saline.
Group B: 25 patients with fentanyl 10 mc/kg (Caspian, Iran) + nitroglycerin 500 mc (Caspian, Iran) diluted in 10 mL distilled water and then diluted with 100 mL normal saline.
Group C: 25 patients with fentanyl 10 mc/kg + nitroglycerin 1000 mc diluted in 10 mL distilled water and then diluted with 100 mL normal saline.
The patients at the beginning of the recovery room and prior to putting the pain pump and at the time of 4, 8, 12, 24, and 48 hours later were assessed with visual analogue scale (VAS) to measure pain and Ramsay scale to measure sedation score. Meperidine 0.2 mg/kg was injected into the patient when the VAS score was more than 4. Patients were examined for hemodynamic, systolic blood pressure, diastolic blood pressure, mean arterial pressure, and heart rate in all times (SAADAT ALBORZ B5, Iran).
At each studied time, if the patient has side effects, including a heart rate > 120, heart rate < 50, 90 mmHg < SBP > 190 mmHg, nausea and vomiting more than 3 times, and severe headache, the patient’s PCIA pump discontinued and the patient was excluded. Also, in the event of a ≥ 20% drop in the patient's blood pressure, the infusion was discontinued and it was noted. In case of nausea and vomiting, 4 mg of Ondansetron IV was injected. All patients were blind regarding how to collect and record data as well as the kind of medication used in this study.
3.1. Statistical Analysis
Descriptive results were presented as mean ± SD or percentages. T-test was used to compare means. ANOVA repeated measure was used to compare the mean of quantitative variables over time. P value < 0.05 was considered significant. The data were analyzed using SPSS version 21 software.
4. Results
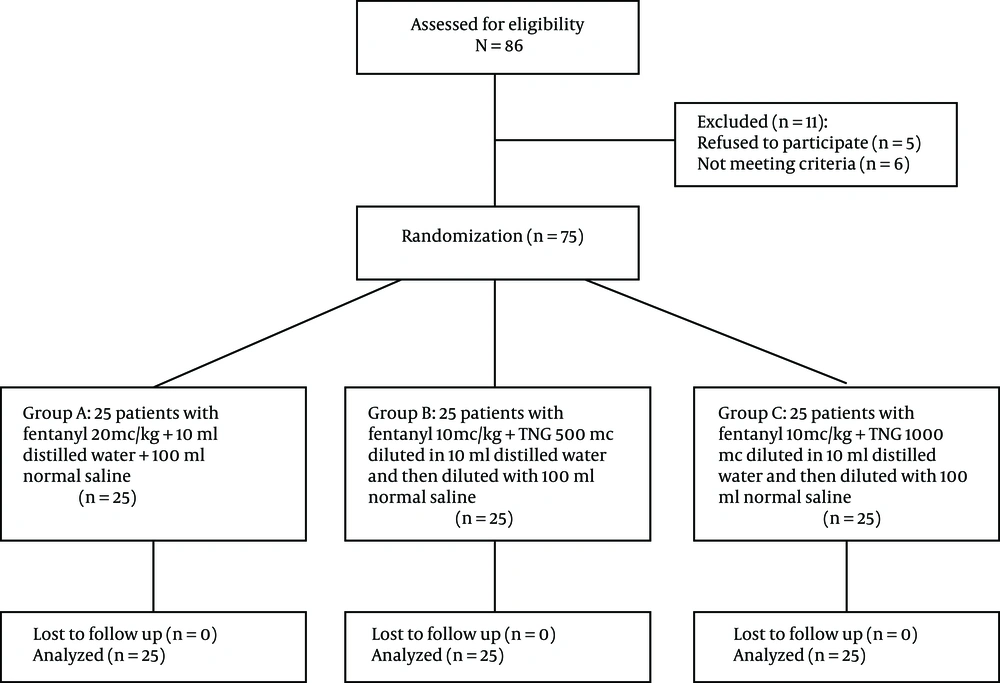
In this study, the weight, gender, and age of the patients in the three groups were compared using one-way ANOVA. The results showed that weight, height, and gender distribution were not significantly different in the three groups (P > 0.05). Table 1 shows the demographic characteristics of the three groups in this study. No statistical difference was shown in terms of operation type (P ≥ 0.05). Figure 1 shows the flowchart diagram of the present study.
| Variable | A (N = 25) | B (N = 25) | C (N = 25) | P Value |
|---|---|---|---|---|
| Age, Mean ± SD | 48.20 ± 17.53 | 49.54 ± 14.93 | 48.28 ± 17.58 | 0.952 |
| Weight, Mean ± SD | 74.48 ± 15.89 | 74.73 ± 9.74 | 72.63 ± 10.72 | 0.821 |
| Gender, No. (%) | 0.577 | |||
| Female | 13 (52.00) | 15 (60.00) | 12 (48.00) | |
| Male | 12 (48.00) | 10 (40.00) | 13 (52.00) |
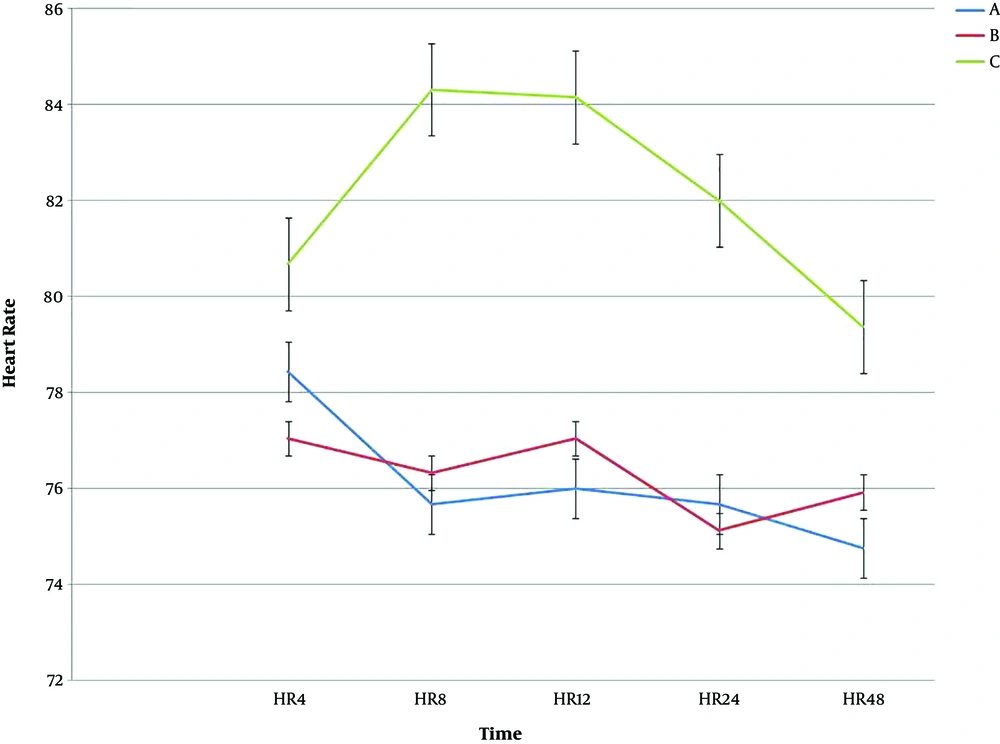
The mean scores of heart rate (HR) for the three groups A, B, and C at 4, 8, 12, 24, and 48 hours after surgery showed that HR at 8, 12, and 24 hours had a significant difference (P < 0.05), but for the 4 and 48 hours after the surgery was not significant (P > 0.05). The results of follow-up tests were as follows: the mean HR in 8, 12, and 24 for group C was significantly higher than group A and B (P < 0.05) (Table 2 and Figure 2).
| Variable | Mean | Std. Deviation | F | P Valuea |
|---|---|---|---|---|
| HR 4 | 0.520 | 0.597 | ||
| A | 78.44 | 10.433 | ||
| B | 77.04 | 17.707 | ||
| C | 80.68 | 7.983 | ||
| HR 8 | 9.196 | < 0.001 | ||
| A | 75.68 | 8.688 | ||
| B | 76.32 | 8.562 | ||
| C | 84.32 | 6.342 | ||
| HR 12 | 7.695 | 0.001 | ||
| A | 76.00 | 7.632 | ||
| B | 77.04 | 9.085 | ||
| C | 84.16 | 7.174 | ||
| HR 24 | 7.586 | 0.001 | ||
| A | 75.68 | 6.650 | ||
| B | 75.12 | 7.384 | ||
| C | 82.00 | 6.752 | ||
| HR 48 | 1.985 | 0.145 | ||
| A | 74.76 | 7.429 | ||
| B | 75.92 | 11.228 | ||
| C | 79.38 | 5.404 |
aP value < 0.05 was considered significant.
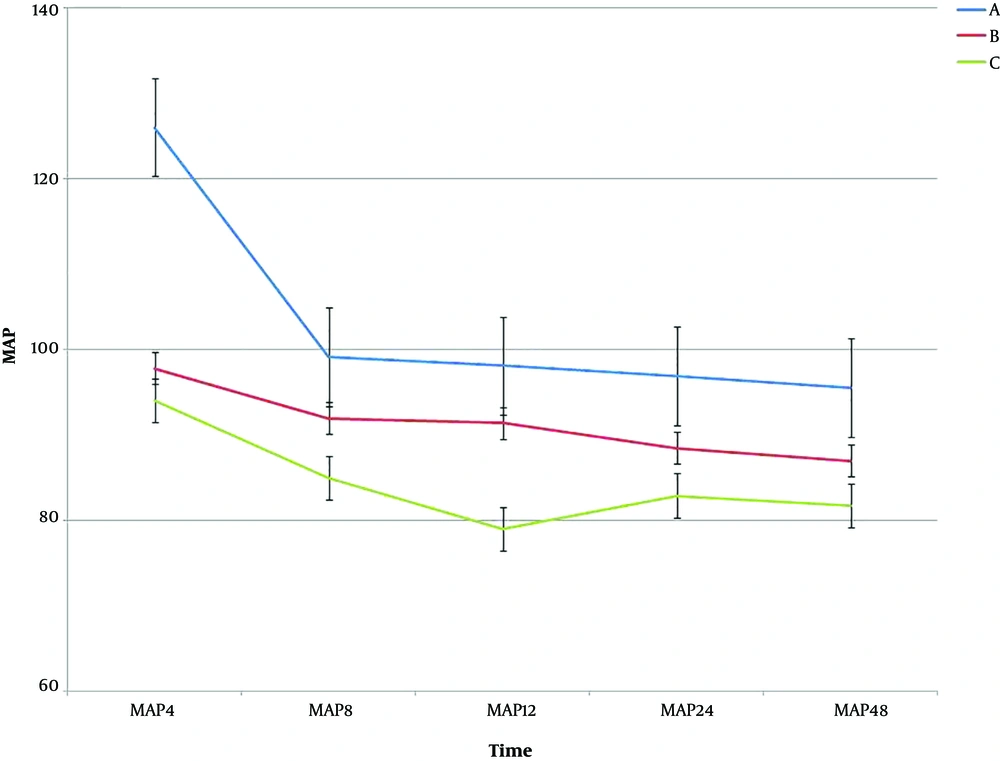
The results showed that there was a significant difference among the three groups at the studied times for HR (P < 0.001). Regarding MAP scores for the three groups A, B, and C, there was a significant difference, except for time 4 and 8 hours after the surgery (P < 0.05). Table 3 shows the results of One-way ANOVA test for the MAP at the studied time. There was no significant difference in the intragroup effects among the treatment groups over time (P = 0.051). In the intergroup analysis, the results also showed that there was a significant difference among the three groups over time (P < 0.001). Figure 3 shows the trend and error bar of MAP in the three groups at different times.
| Variable | Mean | Std. Deviation | F | P Value |
|---|---|---|---|---|
| MAP 4 | 1.49 | 0.231 | ||
| A | 126.04 | 17.53 | ||
| B | 97.77 | 11.12 | ||
| C | 94.06 | 13.65 | ||
| MAP 8 | 11.03 | < 0.001 | ||
| A | 99.12 | 10.64 | ||
| B | 91.94 | 7.03 | ||
| C | 84.98 | 13.29 | ||
| MAP 12 | 24.03 | < 0.001 | ||
| A | 98.09 | 9.98 | ||
| B | 91.38 | 6.88 | ||
| C | 79.00 | 12.46 | ||
| MAP 24 | 14.10 | < 0.001 | ||
| A | 96.90 | 9.88 | ||
| B | 88.46 | 6.32 | ||
| C | 82.90 | 11.24 | ||
| MAP 48 | 8.47 | < 0.001 | ||
| A | 95.52 | 7.87 | ||
| B | 86.97 | 6.83 | ||
| C | 81.76 | 17.83 |
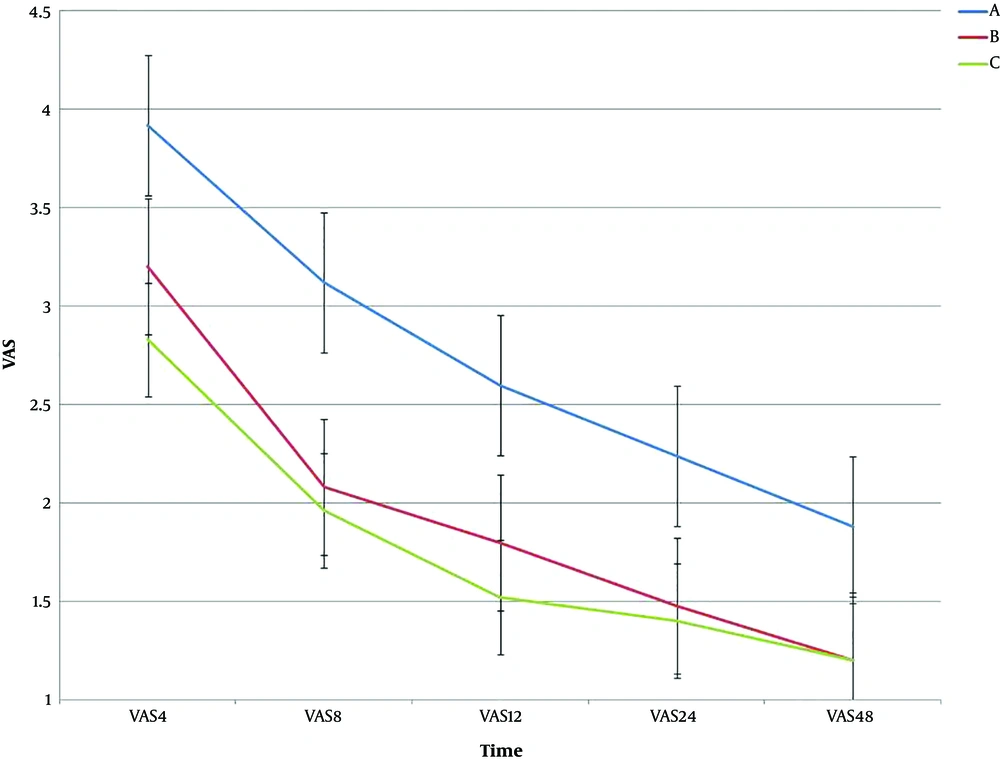
Regarding VAS among the three groups A, B, and C, the results showed that there was significantly different in the three groups (P < 0.05). Table 4 shows the results of ANOVA for VAS at the studied time. For intragroup effects, there was a significant difference between the treatment groups over time (P < 0.001). In the intergroup analysis, the results also showed that there was a significant difference between the three groups over time (P < 0.001). Figure 4 shows the trend and error bar of VAS for the three groups at different times.
| Variable | Mean | Std. Deviation | F | P Value |
|---|---|---|---|---|
| VAS 4 | 4.407 | 0.016 | ||
| A | 3.92 | 1.25 | ||
| B | 3.20 | 1.00 | ||
| C | 2.83 | 1.60 | ||
| VAS 8 | 7.675 | 0.001 | ||
| A | 3.12 | 1.23 | ||
| B | 2.08 | 0.95 | ||
| C | 1.96 | 1.24 | ||
| VAS 12 | 9.709 | < 0.001 | ||
| A | 2.60 | 1.00 | ||
| B | 1.80 | 0.76 | ||
| C | 1.52 | 0.91 | ||
| VAS 24 | 9.971 | < 0.001 | ||
| A | 2.24 | 0.77 | ||
| B | 1.48 | 0.65 | ||
| C | 1.40 | 0.76 | ||
| VAS 48 | 13.442 | < 0.001 | ||
| A | 1.88 | 0.60 | ||
| B | 1.20 | 0.50 | ||
| C | 1.20 | 0.50 |
5. Discussion
This study was designed to evaluate the effects of adding nitroglycerin to the opioid in intravenous analgesia pump for reducing the dose of other analgesics and increasing the efficacy of drugs or reducing postoperative pain. The HR, MAP, and VAS mean scores showed a significant difference in terms of intragroup and intergroup effects in the three groups. Our study shows that pain was lower in patients receiving a higher dose of nitroglycerin (group C). Although the effect of nitroglycerin on hemodynamic was higher in this situation, MAP and HR did not show clinically significant differences among the three groups. We advise the group B nitroglycerin dosage for reducing the pain and less hemodynamic changes during infusion. It has been seen in previous studies that cutaneous nitroglycerine increased the duration of spinal anesthesia during and after the surgery and this drug has not been used via PCIA (4, 6, 7, 17).
In the study of Sen et al., they determine that the addition of nitroglycerine to lidocaine for Intravenous regional anesthesia may improve motor and sensory blockade, decrease tourniquet pain, and better postoperative analgesia without side effects (18). In the present study, the addition of nitroglycerin increased analgesia in the third group, the nitroglycerin dose was higher than the second group, and the mean VAS was lower as well.
In the study of Orbach-Zinger et al., nitroglycerin patches showed no advantage in patients receiving PCA of morphine analgesia after total knee replacement. Therefore, they realized that the safety of using nitroglycerin patch after the surgery must be investigated more (11), this finding was inconsistent with our study and it seems the cause of this difference is the dosage of nitroglycerin in these two studies and underlying disease of studied patients as well.
In the study of Lauretti et al., they found that neither intrathecal 5 microgram neostigmine alone nor transdermal nitroglycerin alone (5 mg/day) postponed the time of first rescue analgesic requirements. The mixture of both providing good postoperative analgesia after vaginoplasty indicated that transdermal nitroglycerin and the intrathecal neostigmine may improve each other's antinociceptive special effects (19). Consistent with our study, nitroglycerin enhanced the antinociceptive effects of fentanyl in the intravenous infusion route by PCA.
Further studies in the future are recommended to investigate nitroglycerin effects as a postoperative analgesic adjuvant via pump infusion or other modalities. In patients with comorbidities such as ischemic heart disease or diabetes mellitus and hypertension, it can be investigated more to see the effects on hemodynamic status because MAP decline can be achieved by adding nitroglycerine in PCIA pump, which could lead to other side effects. Considering this possible problem, we recommend nitroglycerin usage in hemodynamically stable patients.
5.1. Conclusions
According to the results, nitroglycerine, as an adjuvant drug, can be added to the intravenous analgesia pump in patients undergoing surgery for better analgesic purposes. However, we recommend that nitroglycerin should be used in hemodynamically stable patients.