1. Background
Pulsed radiofrequency (PRF) has been widely used in pain treatment since its introduction in 1998 (1). Although minor tissue changes have been observed around the active electrode tip in experimental work (2), many studies have been published on the subject, most of them confirming the efficacy of PRF, but not elucidating its mechanism (3). However, there is a general opinion that the effects of PRF result from the action of the electromagnetic field during active pulse (4).
Traumatic muscle injury of the mouse leg is a simple model of trauma induction under standard conditions suitable for the study of the influence of PRF on inflammatory pain (5). Notwithstanding, it has also been demonstrated that PRF has effects on other inflammatory markers and other biological tissues such as plant tissues (6-8). The local anti-inflammatory effect of PRF raises the possibility that it may also have a potential general effect on the immune system if the mode of application is modified (9).
Most biological changes associated with inflammatory pain share mechanisms that influence redox imbalance. When there is a predominance of molecules with an electron mismatch in the organic microcosm, reactive oxygen species (ROS) molecules may directly damage vital cell constituents such as lipids, proteins, and DNA (10, 11). This damage may share a common final pathway to form paramagnetic free radical species, and the dynamic equilibrium between the singlet and triplet states of free radicals is modified by the magnetic field (12, 13). These modifications would be able to reduce the formation of the final layer of free radical molecules that participate in the development and maintenance of various diseases of our time, such as diabetes, ischemic cardiopathy, cancer, acute and chronic pain, obesity, and autoimmune diseases (10, 11, 14). A reasonable explanation of how biochemical reactions might be influenced by magnetic fields is based on the spin-correlated radical-pair mechanism (SCRPM) (12, 13). Usselman et al. demonstrated that weak magnetic fields, such as those produced by radiofrequency, influence the relationship between O2- and H2O2 production, resulting in the modulation of redox equilibrium, ROS formation, and cell growth (13).
We hypothesized that the magnetic field created by PRF could modify the behavior of unpaired spin electrons, with the end result that the concentrations of free radicals responsible for tissue inflammation would be reduced.
2. Methods
2.1. Ethics Statement
All animal procedures were approved by the Hospital de Clinicas de Porto Alegre Institutional Animal Care and Use Committee (protocol no. 16-0369). The study was conducted in compliance with Brazilian Federal Law 11.794/2008. All animals were weighed and anesthetized by administration of inhaled isoflurane.
2.2. Design of the Study
Twenty-four male Wistar rats with a mean weight of 300 g were randomly divided into the following 4 groups of 6 animals as follows:
• Control (CO): subjected to handling
• CO+PRF: subjected to PRF
• T: subjected to trauma
• T+PRF: subjected to trauma and PRF.
2.3. Trauma
Trauma was induced by allowing the rod to fall freely from a height of 180 mm at a 90-degree angle onto the left quadriceps muscle (5).
2.4. PRF
Two 22G standard non-insulated needles were placed intramuscularly, one at the extremity of the injured limb and the other one at the shoulder musculature. The PRF was delivered from a custom-made box (Springlife Medical, Utrecht, the Netherlands) generating an irregular variety of PRF with an average pulse width of 2.89 ms and an average frequency of 5.11 Hz, leading to an average duty load of 14.77 ms/s. We aimed at generating electric fields of 200 V/m. We assumed a value of sigma of 0.35. The surface of the plane perpendicular to the current direction was calculated to be 8 cm2 and the measured impedance was 400 Ohm. This resulted in a required voltage of 22 V. At these settings, the equilibrated heat effects at the tip and along the shaft are < 10 C, even assuming a blood flow of zero. The electric field was set 200 V/m, considering an average surface area of 8 cm2. The value of sigma was 0.35. The current required was calculated at between 50 and 60 mA (average 56 mA). This current was calculated using Ohm’s formula: V = I × R. Since impedance of around 400 Ohm was observed, the voltage required was standardized at 22.4 V (0.056 × 400 = 22.4 V).
2.5. Events
On day 1: T+PRF and T groups were subjected to trauma induction.
On day 3: T+PRF and CO+PRF groups received PRF lasting 10 minutes.
On day 6: T+PRF and CO+PRF groups received PRF lasting 10 minutes.
On day 7: animals were killed by anesthetic overdose, and then the quadriceps muscles were removed and placed in 10% buffered formalin.
The homogenate was prepared according to Llesuy et al. (14). The Bradford method was used to determine the concentration of protein (15). Lipoperoxidation (LPO) was evaluated by concentration of thiobarbituric acid reactive substances (TBARS) using Buege & Aust technique (16). The Misra & Fridovich method was used to determine superoxide dismutase enzyme (SOD) activity (17), and the Boveris and Chance Pattern to determine catalase enzyme (CAT) activity (18). Interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α levels were measured using the Milliplex® kit.
2.6. Statistical Analysis
The sample size was calculated to detect differences in oxidative balance with magnitude (effect size: I/O) equivalent to 2.5 standard deviations, with α = 0.05 and power = 90%. Based on the results of previous studies, the sample required was calculated as 6 animals per group, chosen at random. Quantitative variables were expressed as means and standard errors and categorical variables as absolute and relative frequencies. Means were compared between groups by using analysis of variance, followed by the Student-Newman-Keuls test. Pearson’s chi-square test supplemented by adjusted residuals was used for comparison of proportions. The Kruskal-Wallis test and the Dunn test were used to compare the sums of histological scores between groups. The significance level was set at 5% (P < 0.05). All data were analyzed by SPSS software, version 21.0.
3. Results
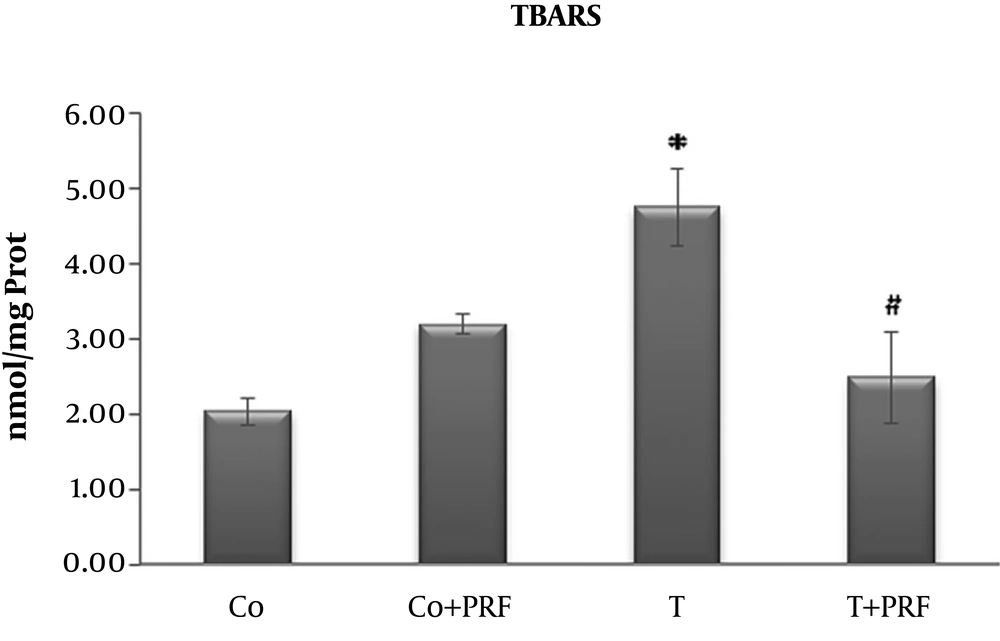
Animals subjected to trauma exhibited a significant increase in LPO in relation to the control group. When PRF treatment was administered to a group that had previously undergone trauma, the indicator returned to values similar to the control animals (Figure 1).
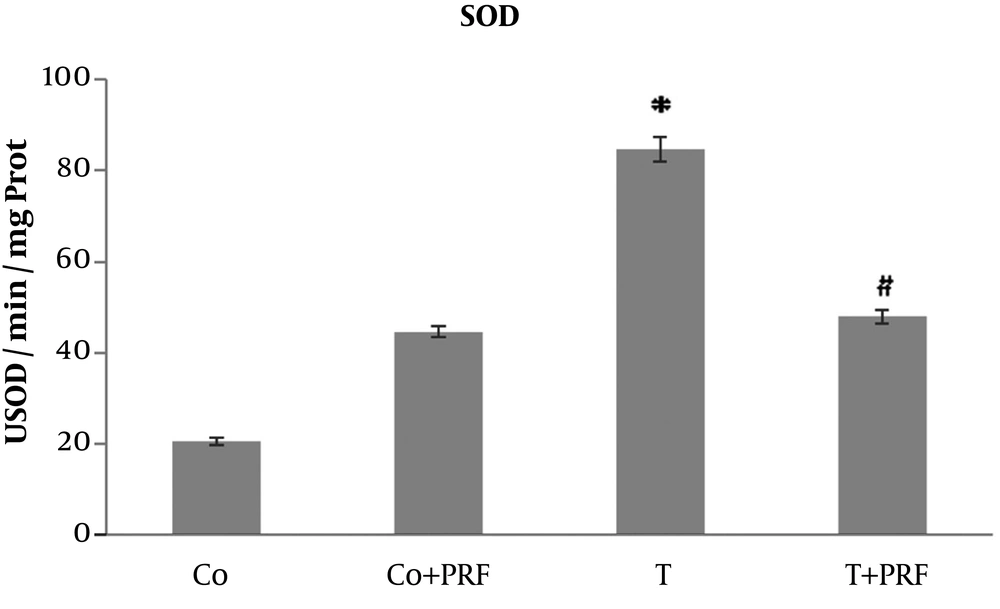
In the T group animals, there was a significant increase in SOD values in relation to control animals. When PRF was administered to the group that had received trauma, a reduction was observed in enzymatic activity, reaching values similar to the control group (Figure 2).
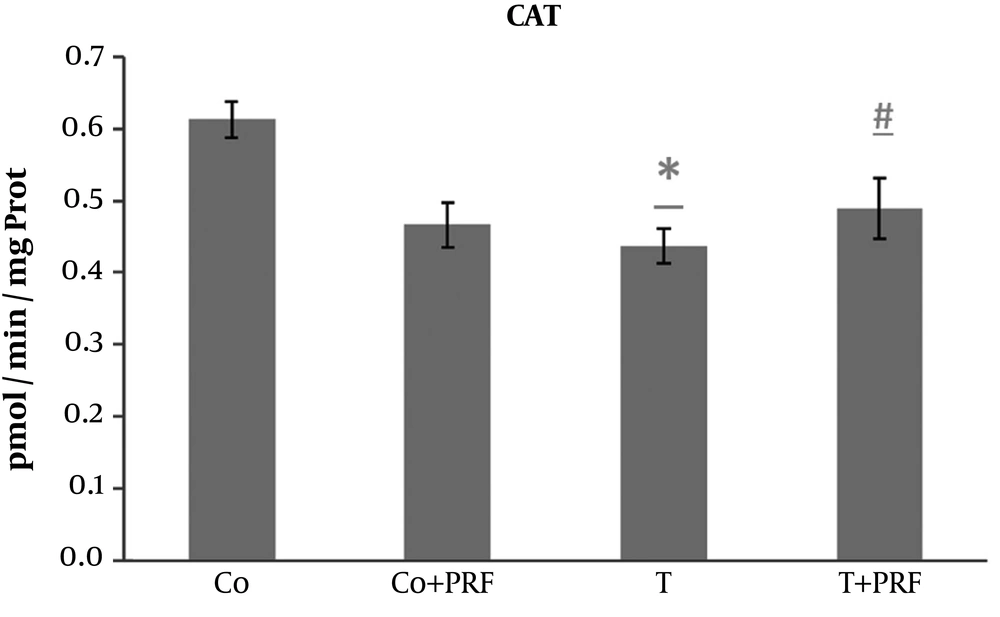
The exposure of animals to trauma caused a significant decrease in CAT compared with the control group. This decrease was avoided when the animals were subjected to PRF administration (Figure 3).
The trauma caused a significant increase in interleukin production: TNF-α, IL-1β, and IL-6. These effects were blocked in the T+PRF group animals subjected to trauma and treated with PRF (Table 1).
| Variables | CO | CO+PRF | T | T+PRF | P Value |
|---|---|---|---|---|---|
| TNF-α | 0.94 ± 0.18A | 1.66 ± 0.26C | 2.03 ± 0.12C | 1.58 ± 0.29B | < 0.001 |
| IL-1β | 5.10 ± 1.57A | 12.4 ± 1.71B | 13.4 ± 1.24B | 3.90 ± 0.60A | < 0.001 |
| IL-6 | 58.7 ± 10.5A | 120.6 ± 26.4A | 191.9 ± 36.1B | 61.7 ± 14.5A | 0.007 |
aValues are expressed as mean ± SE.
bSame superscript letters are not significantly different by the Student-Newman-Keuls test at 5% significance level.
4. Discussion
Since many of the physiological and pathological situations in which PRF has several applications involved in redox balance (12, 18). We hypothesized that these phenomena could be linked. Could redox biology be the common denominator that explains effects in so many different biological tissues?
The redox balance is nothing more than maintenance of the electrogenic stability of tissues through control of excess reactive species by the normal organic antioxidant defenses, including CAT and SOD (10, 11). A predominance of molecules with mismatched electrons in the organic microcosm (as we reproduce in the model of inflammatory pain) also triggers extremely destructive chain reactions similar to that occurring with LPO, leading to potentially serious tissue damage (10, 11). This imbalance is called oxidative stress and is present in diseases with high prevalence and morbidity in the Western world and include chronic pain, diabetes mellitus, heart, liver, neurological and degenerative diseases, rheumatologic autoimmune disorders, and cancer (10, 11, 14).
In our study, oxidative stress was produced by muscle trauma in a nociceptive pain model with an inflammatory process. We demonstrated that LPO levels detected by the TBARS technique were higher in the T group and were significantly reduced in the T+PRF group, suggesting reduced LPO, i.e., less oxidation of long-chain fatty acids, resulting in a protective effect on biological membranes from PRF administration.
The SOD disrupts O2- and forms H2O2 through oxidation and reduction processes, controlling the steady-state concentration of O2 (10, 11, 14). We observed a significant increase in SOD enzyme activity in the T group compared to the control group. This effect probably occurred as an adaptive response to excess reactive species. After administration of PRF (T+PRF group), the antioxidant SOD enzyme values decreased to values similar to those in the control group. Since CAT is a holoenzyme that catalyzes dismutation of H2O2 to form O2 and H2O and is located primarily in peroxisomes, it removes the H2O2 normally generated in β-oxidation reactions of fatty acids or oxidation of alkanes and thus the levels may be altered in situations of oxidative stress (10, 11). Our study showed the changes since its levels were reduced in animals in the T group. This decrease in CAT may be a response to the oxidative stress that increased SOD activity, generating greater quantities of H2O2, which is a situation that would require greater consumption of this enzyme to remove it, leading to reduced levels (10, 11, 14). The PRF was associated with restoration of CAT values to near the baseline level. The significant response of CAT in the T group suggests adaptive antioxidant defense mechanisms, which may contribute to preventing increased muscle LPO in this group (10, 11, 14).
Moreover, TNF-α is involved in inflammation in general and has certain biological effects, such as activation of macrophages and neutrophils and increased adhesion molecules involved in leukocyte rolling, cell differentiation, and apoptosis (5, 14). The trauma caused a significant increase in TNF-α production. This increase was consistent with establishment of the traumatic inflammatory injury. These effects were blocked in the animals in the T+PRF group that underwent trauma and were treated with PRF.
On the other hand, IL-1β promotes the migration of leukocytes to sites of injury or infection and is usually produced by monocytes and macrophages. Its induction and consequent production may lead to increased expression of endothelial adhesion molecules and stimulation of IL-6 and TNF-α production (5, 14). In our study, the trauma occurred with a significant increase in IL-1β production. This increase was significantly lower in the group of animals subjected to trauma and treated with PRF.
In addition, IL-6 plays an important role in homeostasis of the immune system and secretion can be induced in different inflammatory conditions by activation of toll-like receptors, lipopolysaccharide, and TNF-α (5, 14). In the current study, IL-6 production indicated a statistically significant increase in animals subjected to the traumatic injury model, in relation to the control group. After receiving treatment with PRF, animals previously injured by the trauma exhibited a decrease in IL-6 production, reaching a value similar to that of the control animals.
In summary, PRF was able to reverse oxidative stress, inflammatory modifications and changes in antioxidant enzymes induced in muscle by trauma. But how can a weak magnetic field affect biological tissue and its biochemical reactions? The dynamic equilibrium between the singlet and triplet states of free radicals may be influenced by weak magnetic fields, such as those produced in tissues with PRF administration, and the reaction kinetics thus become magnetic-field dependent (12, 13, 19, 20).
4.1. Conclusions
Our findings identify modifications in a biological system provoked by magnetic field that may be the common denominator needed to explain many effects of PRF in different biological tissues. It is possible that PRF acts on radical-pair magnetic sensors, affecting singlet-triplet transitions, thereby exerting its therapeutic effects as a stabilizer of redox balance. Nevertheless, more studies are needed to better elucidate the relationships between the events described here.