1. Background
In 2005, the World Health Organization (WHO) indicates that there are 1.6 billion and 400 million obese people whose body mass index is above 25 kg/m2 and 30 kg/m2. According to this report, the prevalence of overweight and obesity in Iran among the demographic profile of 15 - 30 years old people is 22% and 16%, respectively (1). Adipose tissue, generally known as fat, plays a major role in regulating energy homeostasis, insulin sensitivity, carbohydrate, and lipid metabolism (2). The adipose tissue is the largest endocrine organ which exudes hundreds of hormones and cytokine. It affects the processes in the local and environmental systems. Also, it produces a variety of pro-inflammatory and anti-inflammatory hormones, including resistin, visfatin, adiponectin, as well as other cytokines, such as interleukin 6, and Tumor Necrosis Factor-α (3, 4).
Primarily made in adipose tissue, resistin is a peptide hormone that contains 108 amino acids including cysteine (5). The level of resistin is high in fat people with cardiovascular disease (6). It has a direct relationship with the risk of arteriosclerosis and C-reactive protein levels (6). It increases the risk of arteriosclerosis through disrupting glucose and lipid metabolism. Moreover, it enhances the vulnerability of platelets in atherosclerosis by stimulating the pro-inflammatory cytokines (7). Resistin can also be considered as a link between obesity, diabetes, and insulin resistance. Some of the results indicate that plasma levels of resistin will increase in obese people (3).
There is a relationship between obesity and increased levels of visfatin (8). Known as phosphoribosyl transferase, visfatin is a 25 kDa adipokine which exudes from adipose tissue; it has an insulin-like function, stimulating the glucose uptake in the adipose tissues and myocytes. Visfatin blocks the release of glucose from the liver and is accompanied by weight loss and reduced waist-hip ratio (9).
The importance of exercise-induced resistin and visfatin is recognized as a therapeutic goal for obesity. However, changes in the level of blood resistin and visfatin following various exercises are not fully evaluated (10, 11). The amount of resistin and visfatin concentrations is dependent upon the intensity, duration, and type of exercise. Therefore, short term training, resistance exercises, and high-intensity exercises can have different effects on reducing resistin and visfatin concentrations compared to the low-intensity exercises and aerobic exercises (12-15).
Abedi et al. evaluated the effects of aerobic exercise with 55 to 65% intensity of heart rate reserved in resistin levels and insulin sensitivity during eight weeks (3 sessions per week). They concluded that there was a significant decrease in resistin levels, BMI, and insulin resistance (12). On the other hand, Jorge et al. reported that after aerobic exercise during 12 weeks there was no significant change in resistin levels and insulin resistance (16). Ghafari et al. studied the effect of continuous training during eight weeks (20 minutes activity, 60% of maximum speed) and high-intensity interval training (2 minutes activity, 80% of maximum speed; 2 minutes recovery, 30% of maximum speed) on resistin and insulin levels, and insulin resistance in male Wistar rats with diabetes mellitus type 2. They concluded that interval training would lead to significant changes in all the factors except resistin gene expression. There was a significant relationship between changes in insulin resistance and resistin gene expression (13).
Exercise is considered as an effective therapeutic method to reduce metabolic diseases, including obesity. According to the limited studies concerning the effect of exercise on resistin and visfatin levels and incompatibility of findings due to different training methods, and aerobic exercise as an effective exercise for the prevention and treatment of obesity, it is necessary to examine the various intensities of exercise, especially in obese people. Therefore, the present study compared the effects of aerobic training with various intensities on obese male Wistar rats in order to examine which of the above-mentioned intensities of aerobic exercise (various intensities or both of them) can probably stimulate the changes concerning the serum levels of resistin and visfatin. Therefore, it was carried out to determine the effect of moderate and high-intensity aerobic exercise on the serum levels of resistin and visfatin in obese Wistar rats during eight weeks.
2. Methods
2.1. Animals
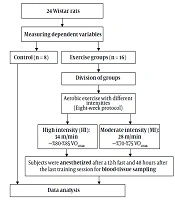
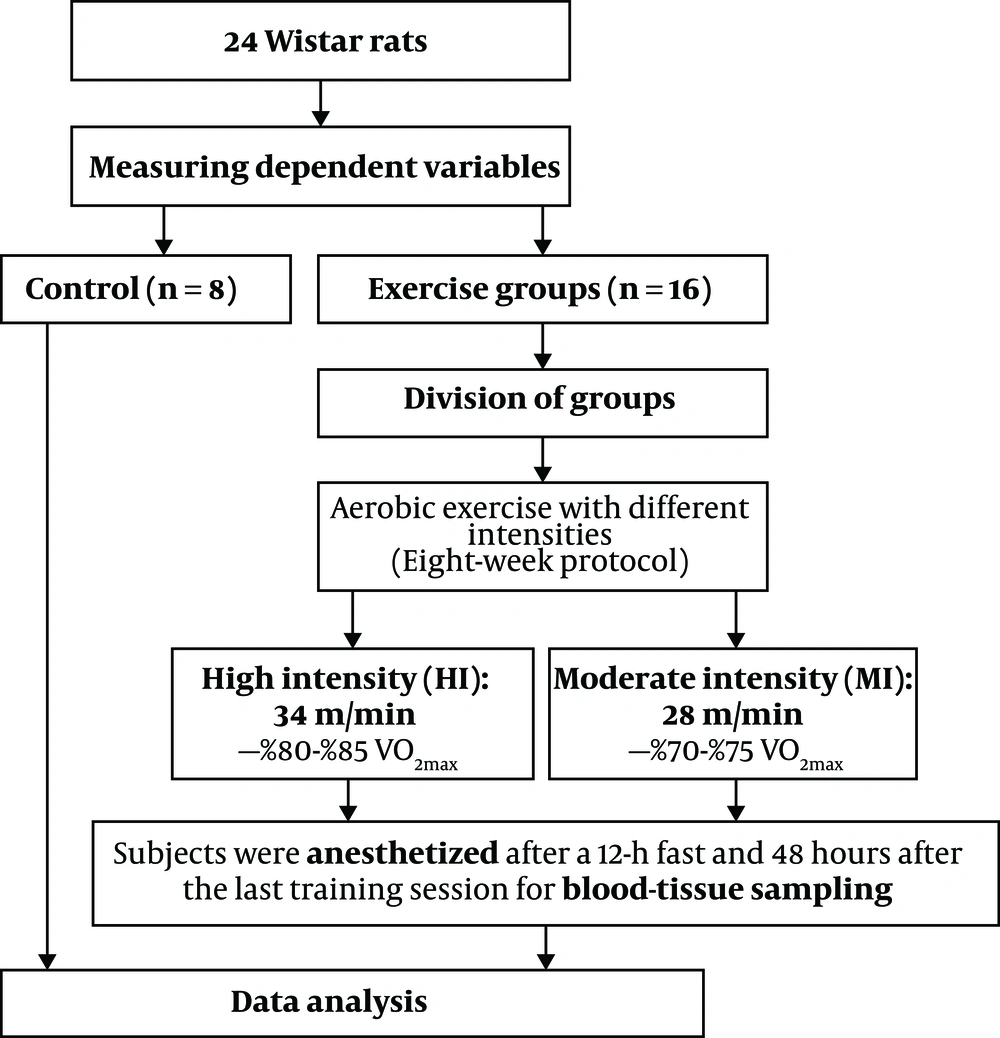
A total of 24 healthy male Wistar rats (eight-week-old, 250 to 300 g weight with body mass index (BMI) > 30 g/cm2) were purchased from Razi Vaccine & Serum Research Institute of Iran and transferred to animal science laboratory of Physical Education and Sport Sciences at Ferdowsi University of Mashhad, Iran. They were kept for 2 weeks in a cage made of polycarbonate under controlled conditions, i.e. average ambient temperature 23 ± 1°C, humidity 50 ± 3%, and 12-h light/12-h dark cycles with free access to ad libitum food and water. The cage was disinfected 3 times a week with 70% alcohol. After two weeks of familiarity with the lab environment, animals were randomly divided into three groups: (1) moderate-intensity aerobic exercise (n = 8), (2) high-intensity aerobic exercise (n = 8), and control (n = 8) groups. It should be noted that the principles of Helsinki Declaration and the opinions of the Ethics Committee of Research were respected at all stages of the research. Also, the steps for tests were approved at the Ethics Committee of Medical Research at the Faculty of Veterinary Sciences of Ferdowsi University of Mashhad under the Code IR.MUMS.REC.2016.131 at Ferdowsi University of Mashhad.
2.2. Induced Obesity Rat
The obese group ate a high-fat diet, containing more calories and fat than standard food (energy of 4.8 vs. 3.9 kcal in g and fat of 39 vs. 3.5%). All rats were fed this way for 14 weeks and had free access to water and food throughout this phase of the study (Table 1).
| Contents | Protein | Fat | Carbohydrate | Fiber | Ash | Calcium | Phosphorus | Salt | Humidity | Lysine | Methionine | Methionine + Cysteine | Reunin + Tryptophan | Other Materials | Kcal Energy in Grams |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High fat (%) | 18 | 39 | 20 | 2 | 1 | 1 | 0.7 | 0.5 | 5 | 1.15 | 0.33 | 0.63 | 0.95 | 10 | 4.8 |
| Standard (%) | 20 | 3.5 | 25 | 14.5 | 10 | 1 | 0.7 | 0.5 | 10 | 1.15 | 0.33 | 0.63 | 0.95 | 11 | 3.9 |
High-Fat and Standard Food Content
2.3. Obesity Criteria
The obesity criteria in Wistar rats were body mass index (BMI). The BMI of normal rats is 0.45 to 0.68 g/m2 (17). At the age of 14 weeks (at the end of the obesity phase and before the training) the amount reached 0.84 g/cm2.
2.4. Familiarization Stage and Exercise Protocol
The training groups exercised on a rodent motor-driven treadmill for 60 min/day, 5 days/wk for 8 weeks (Table 2). After an acclimatization period to the aerobic exercise protocol, they were placed on the treadmill to walk at a speed of 10 m/min with a 0° slope for 15 minutes for one week. During the second and third weeks, the speed and duration of the exercise were gradually increased, so that the final speed of running of a moderate-intensity group on the treadmill was 28 m/min ∼ 70% - 75% VO2max and the speed of high-intensity group on the treadmill was 34 m/min ∼ 80% - 85% VO2max. The exercise volumes in moderate- and high-intensity groups were 11.76 km/week and 14.28 km/week, respectively (18). Upon the completion of the exercise program, the speed of the treadmill was reduced gradually until it reached zero in order to cool down. The entire exercise protocol is illustrated in Figure 1.
| Groups/Weeks | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| MI | ||||||||
| Duration (min) | 15 | 27 | 34 | 40 | 46 | 52 | 58 | 60 |
| Speed (m/min) | 10 | 15 | 20 | 21 | 23 | 24 | 26 | 28 |
| HI | ||||||||
| Duration (min) | 15 | 27 | 35 | 45 | 54 | 59 | 60 | 60 |
| Speed (m/min) | 10 | 15 | 20 | 22 | 24 | 27 | 31 | 34 |
Moderate and High-Intensity Aerobic Exercise Protocol on Treadmill
2.5. Sample Collection
48 hours after the last exercise session and 12-h of fasting, the rats of all groups were transferred to the laboratory and sacrificed. At first, animals were anesthetized in a special sampling space (sterile environment) by a combination of Ketamine (30 - 50 mg/kg of body weight) and Xylazine (3 - 5 mg/kg of body weight). After confirmation of anesthesia, 5 mL of blood was taken by syringe from the right ventricle of each rat and immediately poured into non-anticoagulant test tubes (19). The blood samples were centrifuged at 3000 rpm for 15 minutes at 4°C. Resistin and visfatin levels were measured by a special Rat ELISA Kit (EASTBIOPHARM, licensed by the United States at No: CK-E91412 & Intra-Assay: CV < 10%).
2.6. Statistical Analysis
The data are presented as mean ± SD. The collected data were analyzed by SPSS 16.0 (SPSS Inc., Chicago, IL, USA). After ensuring the normal distribution of the data, using the Shapiro-Wilk statistical test and the homogeneity of variances by Levene’s test, Paired-sample t-test and one-way analysis of variance ANOVA with Tukey's post hoc test was used to evaluate the differences between groups. Furthermore, the significance was established at the alpha level P < 0.05.
3. Results
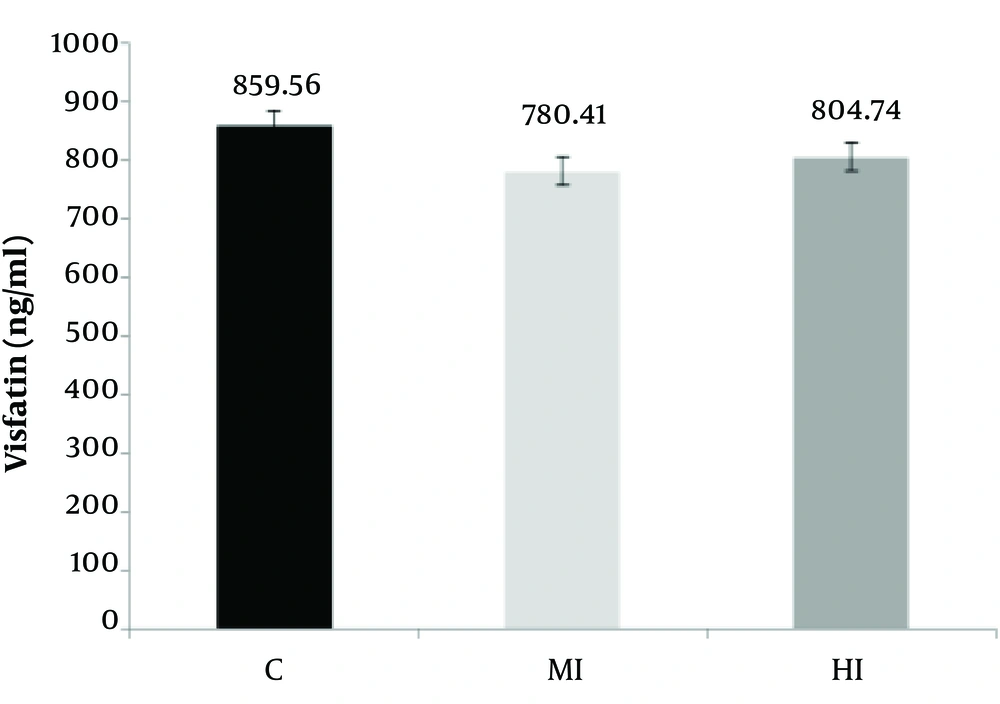
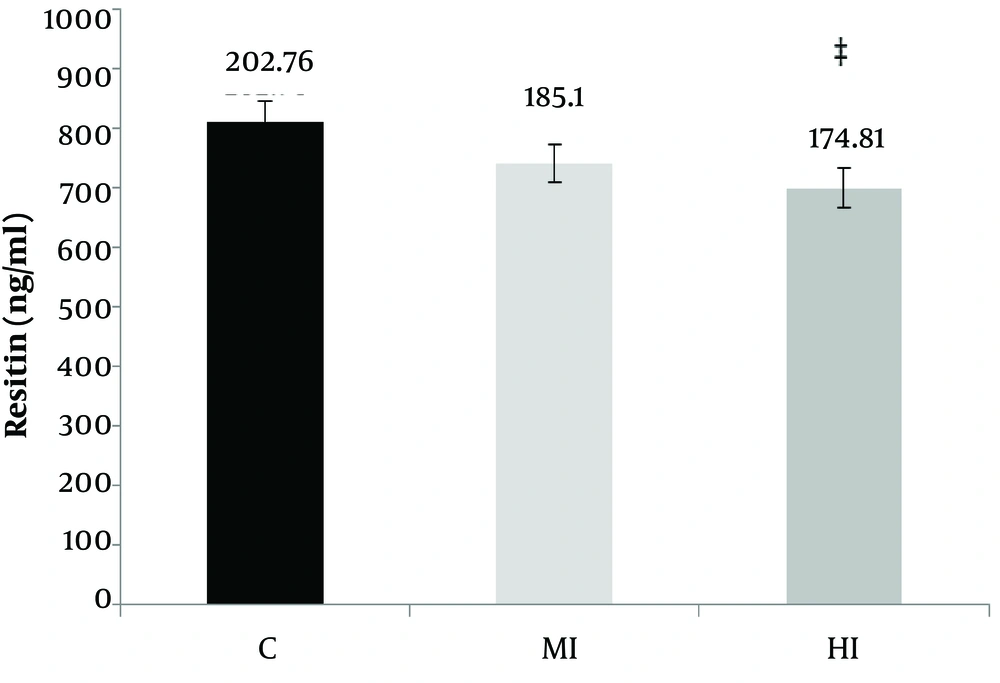
According to Table 3, a significant difference was observed between moderate, high-intensity aerobic exercises and control groups in the serum of resistin concentration (P = 0.001 and F = 3.00). Also, the levels of serum visfatin did not change between moderate, high-intensity aerobic exercises, and control group (P = 0.18 and F = 1.68) (Figure 2).
According to the results of Tukey's post-hoc test, no significant difference was observed in intra-group mean changes between the moderate-intensity aerobic exercises and control group in terms of the resistin concentrations (P = 0.05). But a significant difference was observed between high-intensity aerobic exercises with control groups (P = 0.01). In addition, no significant difference was observed in the visfatin serum mean changes between the moderate-intensity aerobic exercises with the control group (P = 0.14) and between high-intensity aerobic exercises with control group (P = 0.42) (Figure 3). According to Table 4, the bodyweight of rats increased in the control group (P = 0.001) after eight weeks aerobic training. The significant difference in the means reduces both the training group’s moderate- intensity and high-intensity training with control groups in the variables of weight (P = 0.01). According to the results of Tukey's post-hoc test, the intra-group differences in mean body weight were significantly different between the moderate-intensity and control groups (P = 0.001) and between the high-intensity and control groups (P = 0.001).
4. Discussion
The purpose of this study was to compare the effect of aerobic training with different intensities on serum levels of resistin and visfatin in obese male rats. Based on the results of the present study, a significant difference was observed between moderate, high-intensity aerobic exercises and control groups in the serum of resistin concentration. These findings were consistent with the results of Halanin et al. and Duzova et al. (20, 21); however, they were inconsistent with the findings of Hornik et al. and Vardar et al. (22, 23). Despite weight loss in obese rats, the changed serum resistin levels were observed in other studies (24). The roles of endocrine glands are important on adipose tissue, known to secrete large amounts of hormones. Among the hormones, resistin is a new signal molecule that reduces the adipogenesis process. It is expressed in white adipose tissue in females with the highest levels in adipose tissue (25). Studies have shown that resistin is a hormone secreted by adipose which is positively associated with body composition index, such as weight, body mass index, and body fat mass (26). There was a significant association between the levels of resistin, obesity, and insulin resistance (27). There is also a direct relationship between changes in resistin values with changes in body mass index, body fat, glucose, and insulin in obese individuals (28). Possible mechanisms of resistin reduction include pro-inflammatory cytokine changes, such as interleukin-1, interleukin-6, and donor tumor necrosis factor-alpha. If these cytokines are reduced, the levels of resistin will decrease (29).
There are conflicting results about the effect of regular physical activity on TNF-a levels (30). Changes in resistin levels can be attributed to cytokines. It is also produced in humans in addition to adipose tissue in lymphocytes and leukocytes (31).
Based on the results of the present study, aerobic exercise program with moderate and high-intensity decreased the serum visfatin concentration in obese male Wistar rats compared to the control group, but these changes were not statistically significant. These findings are consistent with the results of Matinhomaee et al. and Hidayat et al. (32, 33) and inconsistent with the findings of Taghian et al., Soltani et al., and McKenzie et al. (34-36). It should be noted that visfatin also acts as a nicotinamide amid phosphoribosyl transferase (Nampt) that is essential in regulating cellular energy as well as in the control of NAD-dependent enzymes (37). Visfatin regulates the intracellular activity of NAD/NADH-dependent enzymes that are critical for glucose-stimulated insulin secretion in pancreatic beta cells (38). Circulating visfatin levels are closely associated with white adipose tissue accumulation and its synthesis is regulated by several factors, including TNF-a, glucocorticoids, interleukin-6, and growth hormone (39).
Investigating the insulin-like function of visfatin indicated that this protein increased the glucose uptake in muscle cells and adipose tissue and decreased glucose production in liver tissue through attachment and activation of insulin receptors in a different location other than insulin binding site (40). It has a pro-inflammatory and anti-apoptotic potential that is essential in inflammatory and infectious diseases (41). The decrease in plasma visfatin concentration after aerobic exercise also strengthens the hypothesis of increased visfatin uptake by subcutaneous adipose tissue. This hypothesis originates from the negative correlation between plasma visfatin concentration and its expression in subcutaneous adipose tissue (42). Another factor affecting visfatin depletion is growth hormone secretion which can significantly decrease adipocytes (43). Therefore, for such inconsistent findings may be due to differences in the duration, intensity, and level of training of the subjects.
4.1. Conclusions
The serum concentrations of resistin and visfatin decreased in both of the training groups. However, the level of resistin was reduced significantly in the high-intensity aerobic training group compared to the control group. Therefore, it seems that performing moderate and high-intensity aerobic exercise may have a positive effect on reducing the incidence of obesity-related diseases.