1. Background
The drug delivery system (DDS), based on nanotechnology has significantly improved drug therapy due to inducing pharmacokinetic changes, increasing the duration of action, reducing toxicity, and increasing the half-life of drugs. All of these benefits are possible to reach with targeted drug delivery by using magnetic nanoparticles as carriers.
Drug delivery systems are more efficient than other nanostructures due to their unique properties (1). The effect of nanoparticles’ shape on their catalytic activity is also undeniable, and many studies have synthesized nanoparticles with specific shapes. Nanostructures such as multifaceted nanoparticles that have more available surfaces show much more catalytic activity than nanoparticles that have fewer facets (2, 3). These nanostructures can exhibit high catalytic properties and are widely used in chemical transformation as catalysts (4). Metal nanoparticles can be used in the pharmaceutical industry, and their magnetic properties are employed in the manufacturing of electronic devices and highly sensitive sensors (5-8). Due to the importance of metal nanoparticles and their applications in various fields of experimental sciences, many advances have occurred in recent years in the synthesis and stabilization of these nanoparticles on different platforms, which is essential to prevent adhesion and compaction of nanoparticles (9-11). Curcumin, a polyphenolic compound derived from turmeric, has different pharmacological effects such as anti-inflammatory, antioxidant, and anticoagulant activity. Phase I clinical trials have shown that curcumin at high doses (12 g per day) is safe for humans (12). Therefore, in this study, we developed a relatively simple, rapid, and less time-consuming method for the synthesis of curcumin-loaded chitosan magnetic nanoparticles.
2. Objectives
This study aimed to design and synthesize a modified magnetic nanoparticle as a targeted drug delivery system.
3. Methods
We added 5.838 g ferric chloride hexahydrate (FeCl3.6H2O) and 2.147 g ferric chloride tetrahydrate (FeCl2.4H2O) to deionized water and stirred at 85°C by a mechanical stirrer. A black precipitate of magnetic nanoparticles was formed after adding ammonium hydroxide to the reaction mixture and then dispersed into 80 mg of the prepared chitosan (CS) solution. The diluted compound was then sonicated for 30 min and neutralized with sodium hydrogen carbonate solution. Drops of the aqueous solution of iron-chitosan magnetic nanoparticles were added to nanocurcumin dissolved in acetonitrile. The suspension was then given 24 h to be stirred with a mechanical mixer at 12,000 rpm. This caused nanocurcumin to penetrate the polymer layer of shell-core nanoparticles. The obtained nanoparticles were washed in water to remove unloaded nanocurcumin. The prepared nanostructure was evaluated by Fourier transform infrared (FT-IR) spectroscopy, scanning electron microscopy (SEM), transmission electron microscopy (TEM), and X-ray diffraction (XRD) analysis. Loaded nanocurcumin was measured by a spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) at 450 nm.
4. Results
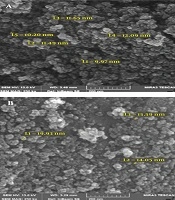
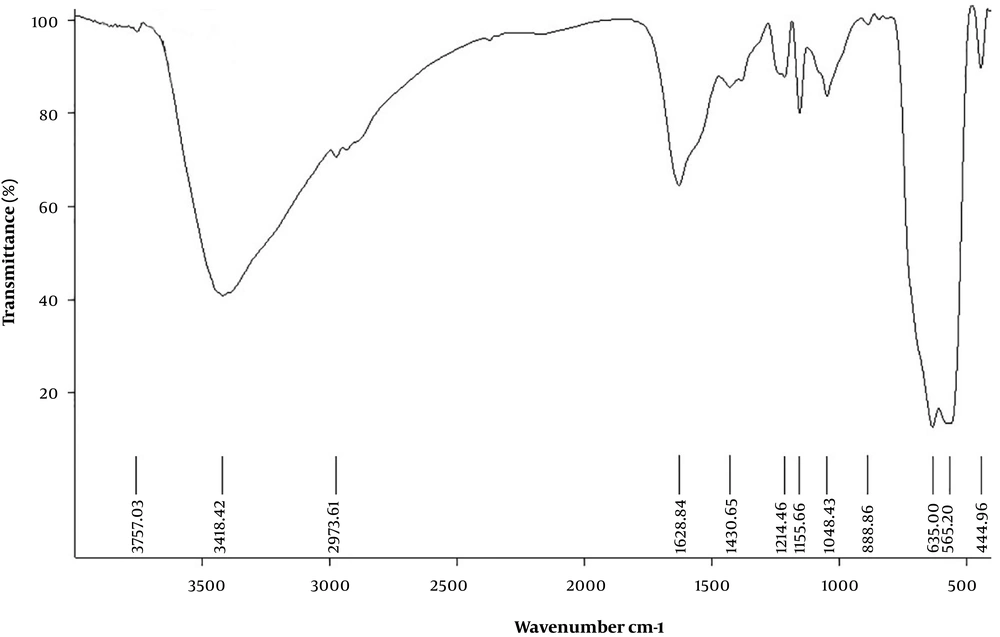
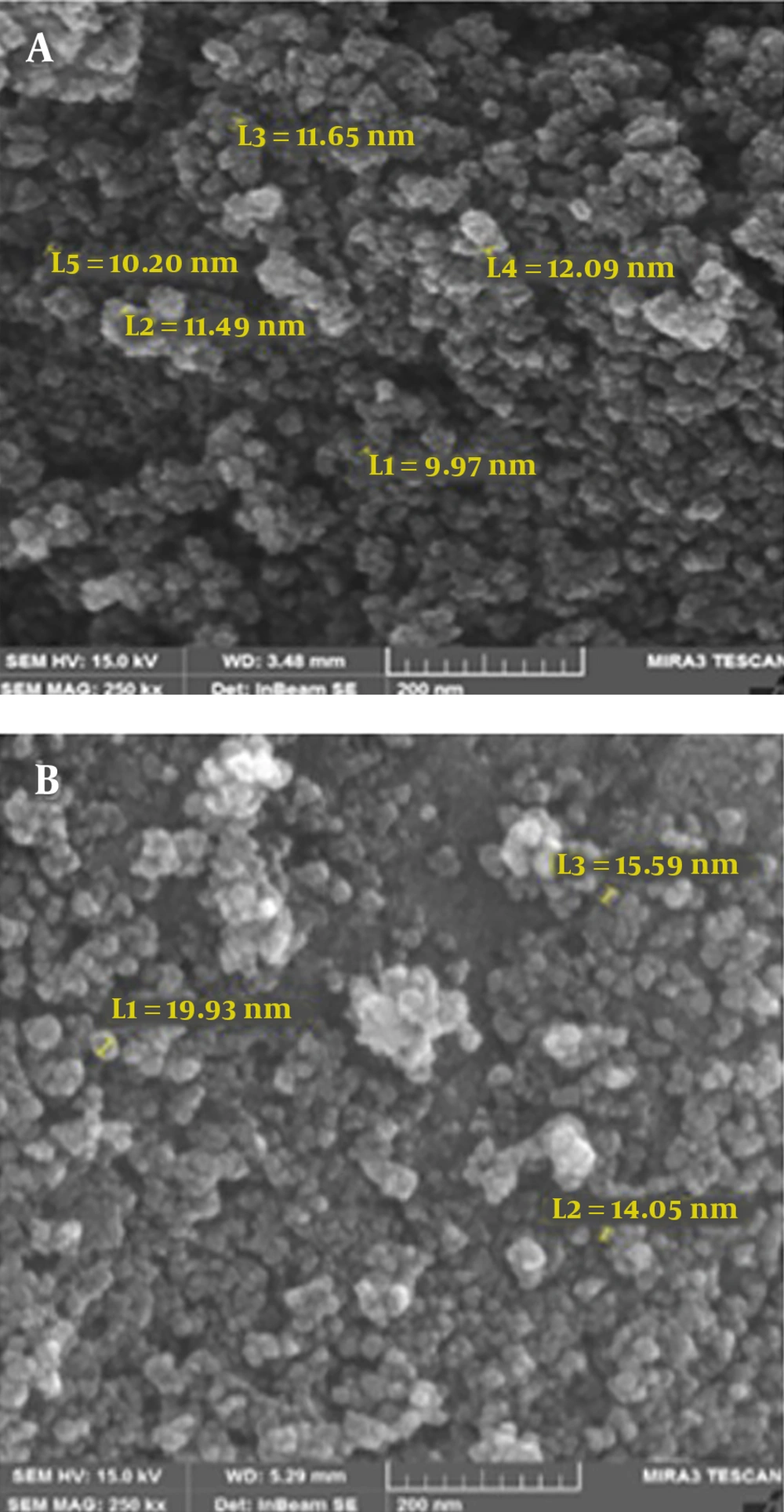
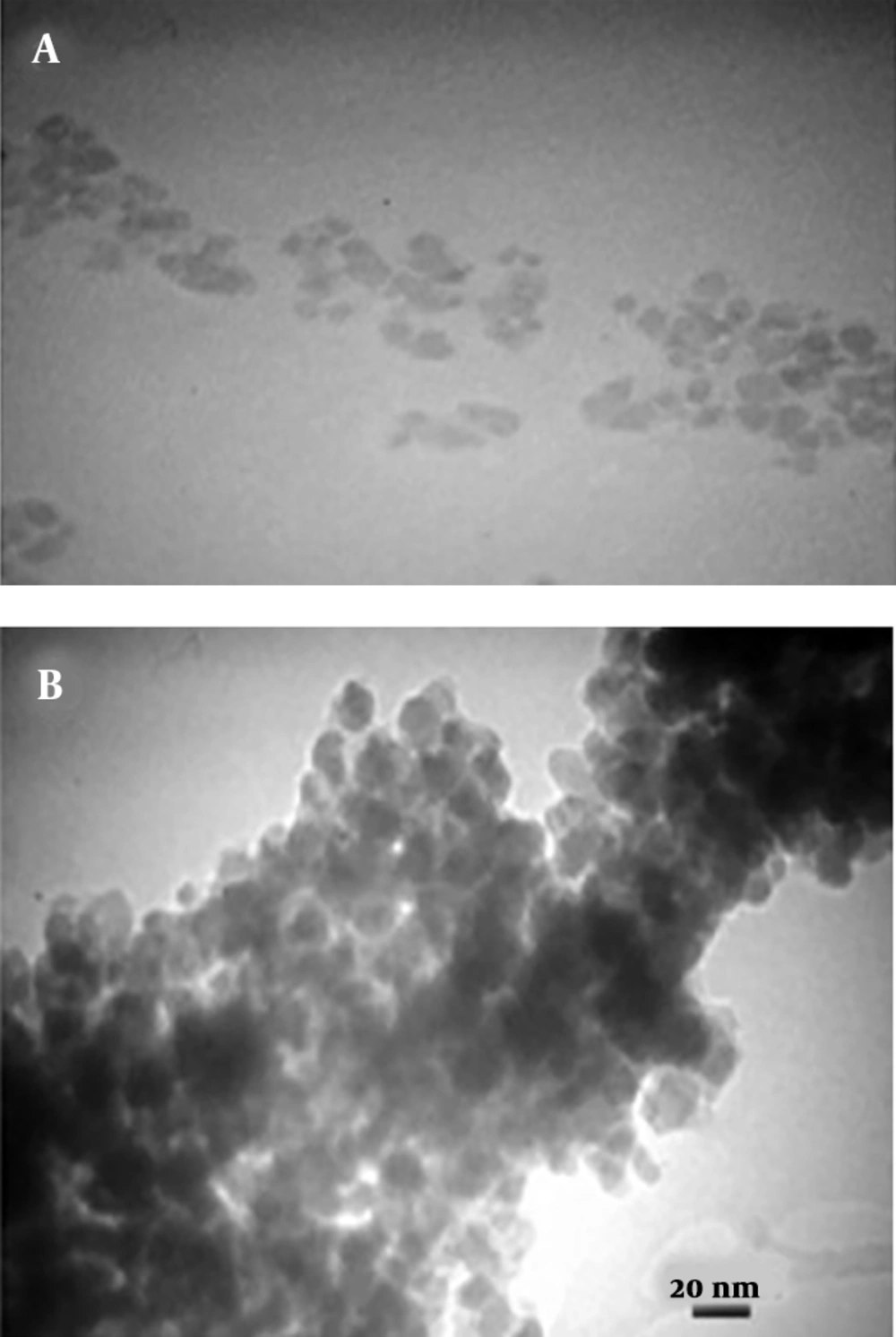
Magnetic nanoparticles of iron oxide were synthesized using ferro/ferric chloride salts, and then their surfaces were coated with chitosan. Finally, nanocurcumin-loaded magnetic nanoparticles with the coat of chitosan were synthesized. The structure of prepared nanoparticles was confirmed by the FT-IR spectrum (Figure 1), SEM (Figure 2), and TEM (Figure 3) images. The FT-IR spectrum showed that nanocurcumin was successfully loaded on the surface of chitosan-modified iron magnetic nanoparticles. The stretching vibration absorption band at 3,421 cm-1 showed the hydroxy bond binding the nanocurcumin layer to chitosan. The absorption bands at 565 cm-1 and 635 cm-1 are related to the iron-oxygen bond in iron oxide. The peaks appearing in 3,400 cm-1 and 2,930 cm-1 regions are related to oxygen-hydrogen stretching vibrations, respectively, in nanocurcumin. The successful stabilization of carbonyl groups was observed with the appearance of an absorption band at 1,628 cm-1. The peak at 1,430 cm-1 is related to the carbon double bond. The carbon-oxygen bond in the chitosan compound also appeared in the 1,155 cm-1 absorption band. The SEM image of the synthesized iron-chitosan magnetic nanoparticles loaded with nanocurcumin confirms that nanocurcumin was made up of uniform particles in nanometer size. The particles were not completely spherical. In addition, particle condensation was observed in some areas, possibly due to the magnetic interaction between particles. Another reason for successful polymerization was the increase in the size of nanoparticles compared to iron oxide nanoparticles (Figure 2). The evaluation of the electron microscopy image of iron-chitosan magnetic nanoparticles loaded with nanocurcumin revealed that the nanoparticles formed a spherical shape on iron oxide nanoparticles containing organic-mineral shells. The size of synthesized nanoparticles is 20 nm, as shown in electron microscopy images. The presence of the polymorphism layer of chitosan nanocurcumin is also shown in the figure.
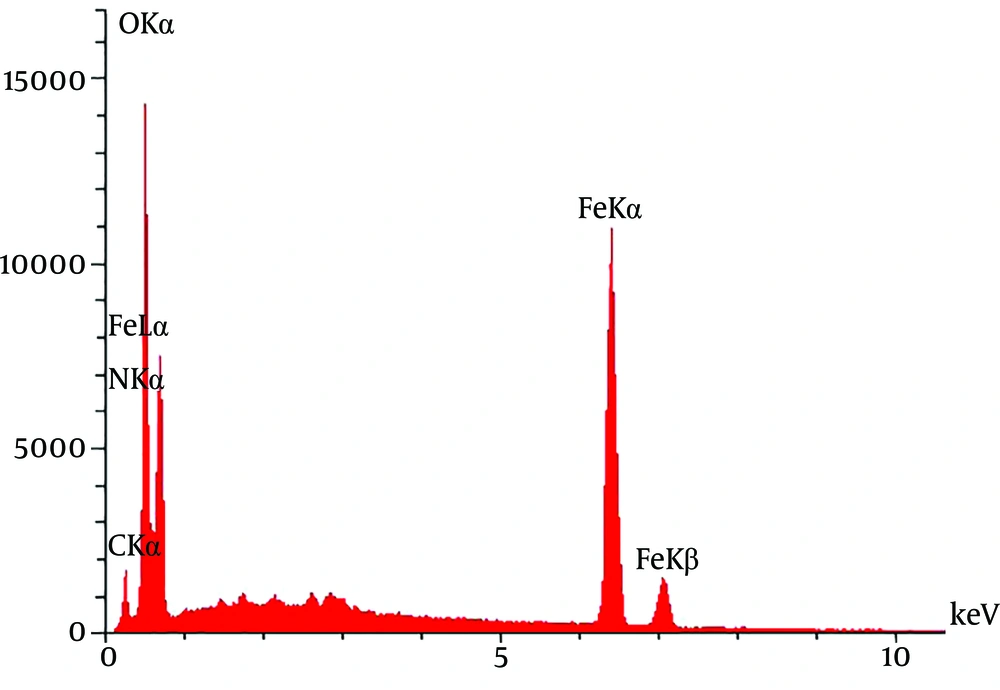
The X-ray diffraction energy spectrum provided qualitative data on the distribution of various chemical elements in iron-chitosan magnetic nanoparticles loaded with nanocurcumin (Figure 4). Moreover, X-ray energy spectroscopy was used to determine the elements in curcumin-loaded nanoparticles on the surface of iron-chitosan magnetic nanoparticles. Iron, carbon, nitrogen, and oxygen peaks confirmed the presence of these elements in the prepared nanoparticles.
5. Discussion
According to Allegra et al. study, curcumin is known as a potent anticancer, antioxidant, and anti-inflammatory agent (13). The study of anti-proliferative and anticancer effects of nanocurcumin by Khaniki et al. (14) showed the benefits of this natural and inexpensive compound for rat’s colon cancer. According to some studies, nanocurcumin inhibits the expression and production of cyclooxygenase-2 (COX-2) (15). One of the most interesting properties of turmeric is its anti-inflammatory properties, so that turmeric is used as a topical and oral medicine to treat inflammation in Chinese medicine (16). The results of our study showed that the synthesized nanoparticles were spherical, and their sizes increased in the process of chitosan coating and curcumin loading, which is a reason for the successful polymerization. Based on the results obtained from the electron microscopy image of iron-chitosan magnetic nanoparticles loaded with nanocurcumin, the nanoparticles formed spherically on iron-oxide nanoparticles containing organic-mineral shells with the size of 20 nm. The magnetic properties of iron-chitosan magnetic nanoparticles loaded with nanocurcumin were determined by a magnetic device, a vibration sample meter, showing that the magnetic saturation of iron-oxide nanoparticles at room temperature was 63.1 emu.g-1 as measured by the magnetic curve (data not shown). A clear decrease in magnetic saturation (35.3 emu.g-1) of iron-chitosan magnetic nanoparticles loaded with nanocurcumin occurred due to the stabilization of polymeric groups on the nanoparticle surface. This evidence confirmed that nanocurcumin had been successfully loaded on the surface of chitosan-modified iron magnetic nanoparticles.
5.1. Conclusions
Nanocurcumin-loaded iron magnetic nanoparticles modified by chitosan were prepared, with active targeting capability. The prepared nanostructures modified by chitosan with appropriate size have a great potential for in vivo applications such as antioxidative, anticarcinogenic, and anti-inflammatory activities.