1. Background
Oxidative stress is caused by an imbalance in the oxidant and antioxidant status of the body. Reactive Oxygen Species (ROS) have a positive role in regulating cell activity (1). The damage caused by ROS after reperfusion has been reported in many different tissues, including the skeletal muscle. Orthopedic surgery increases the possibility for the production of oxidant agents (2).
Inflammation increases neutrophil activity and ROS release (3). There is ample evidence confirming ROS overproduction in the body under pathological conditions such as hypertension, cholesterol elevation, and LDL oxidation (4). The most reliable indicators for measuring oxidative stress are Malondialdehyde (MDA) and Thiobarbituric Acid Reducing Substances (TBARS). Total Antioxidant Capacity (TAC) involves determining the total amount of antioxidants in the plasma rather than measuring the value of each antioxidant individually (5, 6). Oxidative stress is also generated by increasing tissue free radicals such as aerobic metabolism in the mitochondrial respiratory chain (1). Besides, TAC is a decisive determinant of oxidative stress in patients undergoing surgery. Total body antioxidants include the enzymatic endogenous system (e.g., superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase) (7) and the non-enzymatic exogenous system (e.g., ascorbic acid, tocopherol, vitamin A, beta-carotene, and urate) (8). Several antioxidants can convert ROS to some less harmful compounds (9). Superoxide dismutase is a metalloenzyme that breaks down superoxide anion to molecular oxygen and comprises an important part of the cellular antioxidant defense mechanism (10). Glutathione peroxidase catalyzes hydroperoxide reduction and converts GSH to its oxidized form. Moreover, GSSG is reduced to GSH by glutathione reductase (11). Catalase is an active enzyme that reacts with H2O2 and eliminates its toxic effect (9). Each of these antioxidants performs specific activities and usually works synergistically to enhance the body’s antioxidant capacity (12).
Oxidative stress can usually disrupt endothelial cells and increase diseases, as well as the possibility of undergoing anesthesia and orthopedic surgery (13). Osteocalcin and alkaline phosphatase are extremely helpful in the recovery of a variety of fractures, while oxidative stress has a detrimental effect in this regard. The results of a study on systemic oxidative stress in dogs undergoing bone and joint surgery suggested a significant increase in Total Oxidant Status (TOS) and Oxidative Stress Index (OSI) after surgery. It was found that the imbalance in antioxidant/oxidant status and TOS due to trauma and surgery in orthopedic subjects can aggravate disease conditions (14).
Microscopic changes and bone loss may complicate surgical treatment and delay fracture treatment due to the imbalance in osteoblast and osteoclast function. In another study comparing oxidative stress and Mitogen-activated Protein Kinase (MAPK) in granulocytes of people with and without bone fracture, it was observed that granulocytes of the elderly were more sensitive to oxidative stress at times of injury than those of young individuals. Mitogen-activated protein kinases are directly affected by oxidative stress stimuli, and their activation may trigger cellular defense mechanisms. For example, antioxidant gene expression gives rise to SOD, which, in turn, eliminates ROS and dismutase superoxide to hydrogen peroxide and molecular oxygen. After a bone fracture, the activity of Jun N-terminal kinase (JNK) decreases, whereas the activities of ERK1/2 and p38 increase (15, 16). Reactive oxygen species show a paradoxical behavior in biological functions. By improving the immune system and participating in cellular messaging, they can prevent many diseases. However, when redox equilibrium is lost, and antioxidant levels shrink, pathogenesis is exacerbated (17). Based on the above discussion, it is crucial to evaluate the level of oxidative stress and assess oxidants/antioxidants balance in orthopedic patients during the treatment process. The present study takes a step towards fulfilling this objective.
2. Methods
2.1. Study Population
According to the literature (5) and the relevant formula, the sample size was estimated at 13 individuals for each group. Nevertheless, to ensure the adequacy of the sample size and account for possible attrition, 40 individuals were finally enrolled in each of the two groups, based on the following assumptions.
Z 1 - α/2 = 1.96 α = 0.05
Z1 - β = 1.64
S1: Standard deviation of MDA in the case group = 0.14
S2: Standard deviation of MDA in the control group = 0.0013
µ1: Mean MDA in the case group = 0.47
μ2: Mean MDA in the control group = 0.14
The subjects included patients who had been hospitalized (between December 2017 and March 2018) with a long bone fracture at the Orthopedic Ward of Khatam Al-Anbia Hospital in Zahedan. Participants of the control group were matched to include healthy people with no fractures or other chronic diseases based on physician diagnosis. The eligibility criteria included patients aged 20 - 40 years who had been hospitalized at the orthopedic ward due to long bone fractures such as femur, humerus, tibia, radius, and ulna. The exclusion criteria covered patients with underlying diseases such as diabetes, liver, cardiovascular, and renal diseases, addiction, taking mineral supplements, and unwillingness to participate in the study.
2.2. Study Design
The demographic characteristics of the patients (including age, gender, occupation, and education) who were examined by an orthopedic physician and hospitalized were registered. The required permits were obtained, and the participants were informed of the purpose and process of research. Weight was measured in kilograms (with an accuracy of 0.1 kg) with minimum clothing. Height was measured without shoes using a non-elastic meter (with an accuracy of 0.5 cm) placed vertically. The Body Mass Index (BMI) was calculated by dividing weight in kilograms by the square of height in the square meter.
To conduct the necessary tests, 5 ml of blood was obtained from each patient in the case and control groups 72 - 96 hours after the fracture. Blood serum was separated and stored in a freezer at -70°C until the markers of TAC, MDA, and SOD were measured.
In this study, MDA was measured using the modified method proposed by Buege and Aust. Accordingly, trichloroacetic and thiobarbituric acid solutions were used to express the optical absorption of samples (17). Serum TAC was measured using the FRAP method introduced by Benzie and Strain (18). Besides, SOD was determined spectrophotometrically using the Zelbio commercial kit (10).
2.3. Ethical Considerations
After the patients were provided with the required information about the study, recruitment began under the supervision of a specialist among individuals willing to take part in this research. This study was approved by the Ethics Committee of Zahedan University of Medical Sciences (IR.ZAUMS.REC.1396.240) on December 26, 2017, and informed consent was taken from all patients and healthy individuals.
2.4. Statistical Analysis
The obtained data were analyzed in SPSS 21 using descriptive statistics. The mean, standard deviation, and the range of changes of the indices were measured, and the t-test was used to compare the results between the two groups. P < 0.05 was considered statistically significant.
3. Results
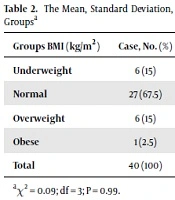
A significant difference was observed between the two groups in terms of total antioxidant capacity (P = 0.003) (Table 1). The MDA values were not significantly different between the two groups (P = 0.09) (Table 1). Similarly, there was no significant difference between the two groups in SOD values (P = 0.2) (Table 1). The mean BMI was 22 ± 3.5 kg/m2 (13.9 - 28.1) in the case group and 23.1 ± 3.97 kg/m2 (14.9 - 32.4) in the control group. The results suggested no significant difference between the case and control groups in BMI (P = 0.09) (Table 2).
| Mean ± SD | Min | Max | P Value | |
|---|---|---|---|---|
| TAC (µmol/L) | 0.003a | |||
| Case group | 748.2 ± 302.83 | 300 | 1626 | |
| Control group | 984.90 ± 207.02 | 337 | 1451 | |
| MDA (µmol/L) | 0.09 | |||
| Case group | 16.61 ± 4.16 | 8 | 26.40 | |
| Control group | 18.45 ± 5.43 | 6 | 70.30 | |
| SOD (µmol/L) | 0.2 | |||
| Case group | 63.41 ± 16.67 | 39.40 | 9.40 | |
| Control group | 58.54 ± 21.83 | 95 | 96.90 |
The Mean, Standard Deviation, and Range of TAC (µmol/L), MDA (µmol/L), and SOD (µmol/L) in the study Groups
| Groups BMI (kg/m2) | Case, No. (%) | Control, No. (%) | Total, No. (%) |
|---|---|---|---|
| Underweight | 6 (15) | 7 (17.5) | 13 (16.25) |
| Normal | 27 (67.5) | 26 (65) | 53 (66.25) |
| Overweight | 6 (15) | 6 (15) | 12 (15) |
| Obese | 1 (2.5) | 1 (2.5) | 2 (2.5) |
| Total | 40 (100) | 40 (100) | 80 (100) |
The Mean, Standard Deviation, and Range of BMI (kg/m2) in the Study Groupsa
4. Discussion
The results of the present study indicated that mean TAC in the case group differed significantly from that of the control group. In line with the current study, Wang et al. reported a significant difference in the TAC index between the case and control groups and observed the occurrence of oxidative stress in orthopedic patients. While oxidative stress may arise before and after fractures in the body, the relationship between oxidative stress and fractures has not been strongly established (14, 19). It has been reported that oxidative stress modulates the differentiation of vascular and bone cells in the opposite direction, which may explain the parallel buildup and loss of calcification in vascular calcification and osteoporosis, respectively (20, 21).
Several studies have proposed that oxidative stress, by increasing isoprostane-F2 in urine and reducing blood antioxidant enzymes, leads to a decrease in bone density and a rise in osteoporosis in older adults (4, 6). The results of the present study implied no significant difference in MDA between the two groups. Post-fracture oxidative stress may be due to an ischemia-induced mechanism triggered by perfusion. During the first three days of fracture, there is a period of ischemia in which no oxidative stress occurs. Subsequently, at the stage of bone formation (callus), in addition to cells’ fibroblast and collagenase, new capillary vessels increase the production of free oxygen as a result of inflammation in other cells. These radicals may cause oxidative damage to the fractured bone, as seen in other tissues with re-injury (5, 22). The appearance of oxidative stress in bone trauma and its deleterious effects on fractures are not unexpected (23). Besides, slightly oxidized low-density lipoprotein increases vascular cell and intracellular oxidative stress and prevents bone cell differentiation (21).
Blood sampling performed just once during the treatment process, and a failure to measure the impact of other oxidant markers on oxidative stress were the two main limitations of the present study, which could account for the lack of a significant variation between the two groups in terms of MDA levels. Gokturk et al. observed that MDA levels amplified significantly seven and 14 days after right tibia fracture. They concluded that oxidative stress occurs in the second and third weeks after fracture. The authors studied the left tibia with no fractures (control) and inserted a pin in the middle part of the spinal cord (case) and, thus, performed a hypothetical surgery. It was shown that inserting a pin into the tibia did not increase the MDA levels. It was also stated that this oxidative stress could potentially improve bone fractures (23).
In another study, urinary isoprostane (iso-PGF2a) was studied as a marker of oxidative stress, and its effect on bone density was investigated. The results revealed a strong biochemical relationship between increased oxidative stress and decreased bone density (4). It has also been mentioned that patients with fractures and no external soft tissue damage could be subject to oxidative stress (5). Concerning the relationship between oxidative stress and bone density in patients with fractures of short and long bones such as limbs or minor fractures, it has been observed that any injury can contribute to the occurrence and aggravation of oxidative stress and thus, require extensive evaluations before and after trauma (4). In another study, Bone Mineral Density (BMD) of the femur was significantly correlated with blood serum MDA. However, the levels of other oxidants were not significantly associated with BMD of lumbar vertebrae (24).
The results of the present study indicated no significant difference in the level of SOD between the two groups, although the case group marked slightly higher in this regard than did the control group. One reliable method of determining oxidative stress is to measure oxidant and antioxidant markers before and after the hospitalization of individuals in the two groups. This will allow for an accurate recording of changes in oxidative stress. Unfortunately, this could not happen in the present research due to its high costs and patients’ lack of agreement for blood sampling in two stages. However, based on a previous finding of the present research regarding TAC and MDA markers, as well as numerous other clinical and experimental studies, it could be inferred that oxidative stress occurs in patients with fractures. Several lines of evidence suggest that, after one or two weeks, the activities of SOD and glutathione reductase (GR) increase considerably in the plasma of patients who regularly receive antioxidants. Administering antioxidant vitamins (A, E, C) and selenium after long bone surgery has improved bone healing and repair. Therefore, it is vital to consider antioxidants in designing postoperative therapeutic protocols for patients undergoing bone surgery (7). The decline in the levels of free radicals in the group receiving antioxidant treatment may be related to the induction of SOD activity, which plays an important role in the generation of superoxide ions produced by osteoclasts (1).
The results of the present study exhibited no significant difference between the BMI of the two groups. Indeed, the two groups were matched based on this variable and other demographic characteristics, as well as the inclusion criteria. Most of the subjects in this study had a normal BMI, and the number of overweight or underweight individuals was limited in both groups. This was because weight and height can influence BMI and are major factors in the anatomy and physiology of bone tissues. This is the case even though the treatment process of bone fracture is predicated based on multiple factors, including the consumption of minerals such as calcium, phosphorus, and magnesium, hormones, and enzymes (25, 26). Oxidative stress has been associated with decreased lower bone density and increased osteoporosis in the elderly. There is a biochemical correlation between the rise of oxidative stress and the decline of bone density.
4.1. Conclusion
The findings of the present study highlighted the increased susceptibility to oxidative stress in orthopedic patients. Yet, this kind of stress can happen before and after experiencing fractures. It is suggested that more extensive studies with larger sample sizes be designed in this field. Oxidative and antioxidant markers are also associated with the homeostasis system, macronutrient metabolism, non-enzymatic antioxidant systems, and some minerals. The limitations of this study were, first, the disagreement of the two groups to take blood sampling twice (before and after treatment) and, second, the high cost of laboratory kits for measuring other oxidative and antioxidant markers. However, to account for the possibility of sample attrition, attempts were made to select a sufficiently large sample size and justify the purpose of research to participants and encourage their contribution.