1. Context
The skin is the largest organ of the body that covers the entire surface and prevents bacteria, viruses, and other exogenous antigens enter the body and water move in and out (1). It is important in a lot of physiological processes like initialization of vitamin D synthesis, regulation of excretion, control of body temperature, and protection from hazardous chemicals and pathogens entry. Severe skin damage can be life-threatening (2). According to the pivotal role of skin in maintaining good health, its integrity is important, and if wounded, its ability to heal quickly and effectively is essential to sustain good health (1). While an appropriate wound healing leads to restoring normal structure, function, and appearance (2), impaired healing can cause chronic wounds, formation of ulcers, and excessive scarring (3); it is also a public health problem (4). Wound healing has a significant economic impact on world healthcare, so that it costs the health system about 28.1 to 96.8 billion dollars per year (5). One of the major health problems is impaired cutaneous wound healing in pathologic situations like diabetes that leads to limb amputations (6). Due to the high prevalence of diabetes, malignant tumors, infections, and vasculopathy, the rate of chronic wounds increases, which imposes a severe economic burden (7). In a pathologic situation, poor wound healing impairs skin regeneration and decreases the quality of life due to pain and immobility (8). Given the importance of wound healing and the fact that not treating open wounds may lead to local infection and ultimately cancer, much research has been done on how to promote wound healing (9).
There are many chemical and herbal medicines and compounds that can help to speed up recovery. The drugs and substances that are used in treatments are the results of research on animal and clinical models. Each intervention that accelerates wound healing has a critical role in preventing health problems due to delayed wound healing; so, a beneficial intervention is necessary (4). Moreover, it is important to consider effective, low-cost, and practical methods of wound healing (10). Many studies have been performed to obtain a new and effective strategy of wound therapies to reduce the costs, provide a long-standing relief, and prevent scar formation (2). Studies indicated that exercise, as a low-cost intervention, is a beneficial strategy in the acceleration of wound healing (5). According to the importance of wound healing in health, this study aimed to review the physiology of wound healing and some effective factors with a special focus on exercise as the complementary medicine.
2. Objectives
This paper aimed to review the physiology of cutaneous wound healing and some negative and positive factors involved in it, with a special emphasis on exercise.
3. Evidence Acquisition
An electronic search without any time limit was performed on scientific databases including PubMed, Google Scholar, and Web of Science. The keywords were ‘wound’, ‘healing’, and ‘exercise’. According to similarities or differences between the results and the relationship with the subject, 53 articles about cutaneous wound healing were selected and reviewed.
4. Results
4.1. Wound Healing Physiology
The skin has regenerative properties to restore tissue integrity (11). Wound healing is a unique mechanism that involves many factors and cells (12). It is a dynamic process and consists of four precisely programmed overlapping phases, including homeostasis, inflammation, proliferation, and remodeling. Each stage must occur in appropriate order, time, and duration. Any disturbance in each phase delays wound healing or leads to non-healing chronic wounds (13). All four phases are interrelated and overlapping (14). Acute wounds pass the normal healing phase, while chronic wounds display a prolonged inflammation phase (2). Since these phases are inter-dependent, success in later phases depends on the success in the preceding phase (1).
4.2. Homeostasis
Homeostasis begins from the onset of injury till several hours after that (3). It happens within 30 minutes post-trauma (15). In this phase, as the first response to injury, the vessels constrict to control bleeding and clot forms. The clot releases growth factors and pro-inflammatory cytokines (13). Platelets and inflammatory cells rush to the injured area and reduce blood flow in the wound bed due to induction of vasoconstriction and release many growth factors like vascular endothelial growth factor (VEGF), epithelial growth factor (EGF), platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), insulin- like growth factor-1 (IGF-1), and interleukin 1 (IL-1) (11), and provide a matrix for cell migration. Production of growth factors is essential for wound repair; for example, VEGF is necessary for stimulating angiogenesis (16).
4.3. Inflammatory Phase
When the bleeding stops, the second stage, which is the inflammatory phase, begins. In this stage, inflammatory cells (neutrophils, macrophages, and lymphocytes) migration to wound initiates (13), and monocytes differentiate to macrophages (3). In a normal wound, inflammation lasts about 2 - 5 days and ends when the harmful agents are removed (3). Platelets release many cytokines and growth factors, leading to an inflammatory response that serves to remove bacteria and other pathogens in the wound (4). Some studies mentioned that the duration of inflammation phase is from the beginning of injury till the third day (17). In this phase, inflammatory cells digest foreign materials and increase vascular permeability (14). In the inflammatory phase, during 24 hours after injury, the number of neutrophils reach to a maximum level but decrease after three days. Within 18 - 24 hours after wounding, the number of macrophages reaches a high level, and on the fifth day, it is the majority of wound cells. Therefore, the inflammatory phase can be divided into early inflammation (24 - 48 hours after wounding) and later inflammation (48 - 72 hours after wounding). In early inflammation, neutrophils and in later inflammation monocytes and macrophages are the main migratory cells, which remove germs (16).
A low level of inflammation is necessary for faster wound healing, but its high level is destructive and delays it (4). Some studies stated the inflammatory phase lasts about 5 - 7 days, which removes contaminating debris and controls microbial infection to initiate repair. Release of inflammatory chemokine (i.e., IL-1 and TNF-α) from the clot and injured cells in the margin of the wound is necessary for the migration of an adequate number of inflammatory cells to the wound area (1). In the inflammatory phase, foreign material and contaminating microorganisms are removed from the wound. The prolonged inflammatory phase leads to chronic and not healing wounds and enters the wound to a chronic state (13).
Oxygen supply is critical for all processes of wound healing like prevention of infection, angiogenesis, migration of keratinocytes, re-epithelialization, proliferation of fibroblast, synthesis of collagen, and contraction. As the result of vascular disruption and oxygen consumption of active cells, wound tissue is hypoxic. Temporary low oxygen and the lactic acid act as signals to stimulate wound healing, production of cytokine and growth factors, initiation of the cell proliferation, migration, chemotaxis, and angiogenesis in the wound bed (13).
Hypoxia stimulates the secretion of cytokine and growth factors from macrophages, keratinocytes, and fibroblasts. Both hypoxia and hyperbaric oxygen produce reactive oxygen species (ROS) (2). A low concentration of ROS disinfects the wound and regulates the signal transduction and gene expression, and facilitates healing (18). The ROS acts as a cellular messenger. For optimum wound healing, an appropriate level of oxygen and ROS is crucial, but excessive ROS damages the wound. Inflammation is necessary for the elimination of contamination, but in its elongation, the wound enters to chronic state (13).
Keratinocytes, macrophages, fibroblasts, platelets, and endothelial cells secrete VEGF that stimulate the formation of new blood vessels. Platelets and macrophages release PDGF and EGF that activate fibroblasts to produce collagen and proliferation (19). Respiratory burst in neutrophils and macrophages release ROS, which kills bacteria, prevents infection and promotes dermal wound repair (20). Low-level endogenous H2O2 supports vascular growth and increases tissue vascularization (16). One effective factor in wound healing is prostaglandins, which have an important role in wound healing through increasing vascular permeability, infiltration and activation of inflammatory cells, and modulation of fibroblasts (21).
About four days after injury, new capillaries form a granular appearance texture, the main components of which include macrophages, fibroblasts, and blood vessels (22). In this phase, the activated complement system secretes vasoactive mediators and chemotactic factors that attract leukocytes to the injury. At this stage, mast cells release histamine and other active amines that produce signs of inflammation around the wound (4). Within 24 hours after injury, neutrophils migrate and begin to clearance infectious agents such as external foreign contaminants, damaged matrix, and dead cells from the wound bed.
On the second day after wounding, monocytes and lymphocytes migrate to the wound, and monocytes differentiate into macrophages that digest necrotic tissue and pathogens and produce growth factors and cytokines (11). Newly formed capillaries supply oxygen and nutrients for growing tissue. Basal keratinocytes initiate migration and proliferation to cover the wound surface. They migrate from the wound margin and proliferate, differentiate, and form a cover over the wound area. Fibroblasts migrate from bone marrow to activate and synthesize collagen, fibronectin, and hyaluronic. The fibroblasts differentiate to myofibroblasts that close the wound surface (11). The secreted cytokines and growth factors lead to an inflammatory response that removes bacteria and pathogens. Macrophages play an important role in killing pathogens, phagocytosis, wound debridement, production of cytokines and growth factors, cell recruitment, and stimulating angiogenesis (4).
4.4. Proliferation Phase
The third stage is the proliferative phase that begins at the end of the inflammatory phase (9). In the proliferative phase, re-epithelialization, neovascularization, and granulation tissue occurs (1). It begins on fourth day and continues until day 21 (3). Some studies reported this phase starts about 2 - 3 days after wounding and lasts until wound closure. In this phase, inflammatory cells and factors are reduced, and fibroblast proliferation, collagen deposition, angiogenesis, tissue granulation, re-epithelialization, and wound closure restructure the wound. Angiogenesis is an essential part of granulation tissue and initiation of re-epithelialization (4). The release of pro-inflammatory cytokines from neutrophils and macrophages plays a critical role in the initiation of the healing cascade (1).
Several days after the beginning of the proliferative phase, capillary grows and granular tissue forms. Fibroblasts synthesize collagen, and re-epithelialization is begun to close the wound (14). The clot and the surrounding tissue of the wound release pro-inflammatory cytokines and growth factors. In the proliferative phase, the epithelial cells proliferate and migrate to cause re-epithelialization. In this phase, fibroblasts produce extracellular components of the matrix-like collagen, glycosaminoglycan, and proteoglycan (13). After seven days, keratinocytes migration is necessary for wound closure. In chronic wounds, epidermal cell migration is impaired, and IGF-1 expression quickens it. IGF-1, by increasing neovascularization, improves chronic wound via VEGF dependent mechanism (23). Nitric oxide, cytokines, and growth factors stimulate re-epithelialization. 2 - 3 days after injury Keratinocytes in the wound border and stem cells from hair follicles and sweat glands nearby the wound cover the wounded surface and secrete proteins to rebuild the basement membrane. Fibroblasts promote wound healing by producing cytokines, chemokine, and growth factors that enhance angiogenesis (3).
4.5. Remodeling Phase
Following the synthesis of the extracellular matrix, when the re-epithelialization is completed, the final stage of wound healing (remodeling phase) begins, which lasts a long time (13). It begins from the end of the granulation tissue formation (24). The duration of remodeling phase is from 21 days until one year after wounding (3). Contraction of the wound occurs throughout the remodeling phase by myofibroblasts (15). Fibroblastic cells transform into myofibroblasts (19).
Fibroblasts synthesize collagen and glycosaminoglycans, which increases steadily until the third week to reach a point of equilibrium, the point that collagen synthesis and breakdown are equal. Fibroblasts synthesize extracellular matrix, which provides a framework for cell migration and proliferation, and increases wound tensile strength (9). In this phase, collagen III exchanges for collagen I (3).
Within 3 - 6 months, the newly formed scar degenerates and changes to soft and mature tissue. In this phase, deposition of the extracellular matrix is more than degradation (19). At the end of the remodeling phase, wound tissue resembles normal tissue (15).
Remodeling phase is involved in restoring tissue structure and function (1). In this phase, the changes in composition and structure of the extracellular matrix increase tensile strength (4). The maximum tensile strength of healed wound is approximately 80% of normal skin (3), and collagen type III is replaced by type I and increases the tensile strength of the tissue to normal. Fibroblasts and other cells secrete matrix metalloproteinase enzyme (MMPs) that degrades extracellular matrix (ECM) and is a key factor in wound repair (11). Excessive proliferation results in scarring or keloid formation (5). Sometimes complete epithelialization of the wound area is not performed and forms a scar, which is an accumulation of fibrotic cells that may limit the normal functions of the organ (25).
4.6. The Effective Factors on Wound Healing
The factors that affect wound healing include enough oxygenation, age, sex hormones, stress, diabetes, obesity, medications, alcoholism, smoking, and nutrition (13). Some of these factors are local that directly affect the wound, and others are systemic and indirectly influence it (9). Some medications (such as steroids), lifestyle (such as sedentary), disease (such as diabetes), and biological situation (such as ageing) delay wound healing (4). Many factors like topical hormones, norepinephrine, hyperbaric oxygen, exercise, growth factors, and nutrition can improve wound repair (4).
Systemic factors such as age, vascular or metabolic diseases, and some drugs may affect wound healing (2). For instance, because of the positive effect of estrogen on wound healing, female mice heal faster than their male counterparts (15). Studies showed that growth factors promote tissue regeneration; however, their usage is not recommended due to their dangerous side effects (25).
Common wound care is categorized into modern and traditional treatments. While traditional-based medicine uses plant compounds such as Nigella sativa and honey to speed up wound healing (5), modern-based treatment uses laser and hydrogel (5). The herbal healing effect is mediated by the anti-inflammatory and antioxidative properties; but they cannot permanently replace the use of current drugs and modern dressings (5).
4.7. Negative Factors on Wound Healing
4.7.1. Medication
Anti-inflammatory and chemotherapeutic medications interfere with platelet function and inflammatory processes and slow down the speed of wound repair. Glucocorticoid, through reducing the expression of inflammatory mediators and chemokines by inhibition of migration of inflammatory cells to the wound area impair wound healing (15). Glucocorticoids, as anti-inflammatory agents, suppress immune cells, modulate Th1-mediated immune responses, and delay the healing process as a result of increasing the duration of the inflammatory phase (26). Topical application of glucocorticoids stimulates the production of inflammatory cytokines and inhibits keratinocyte growth factor (KGF) expression (27).
4.7.2. Biological Situations
One of the risk factors that impairs wound healing is sleep deprivation because inadequate sleeping causes dysregulation of circulatory cytokines. Malnutrition and consumption of heavy alcohol delay wound healing. Also, it is well known that smokers heal wounds more slowly than nonsmokers (1).
Obesity, diabetes, stress, and ageing are accompanied by low-level inflammation, which impairs cutaneous wound healing (6). In elderly and obese individuals, basal levels of inflammation increase (15). In an obese person, adipocytes and macrophages in the adipose tissue produce adiponectin, which has a bad effect on immune and inflammatory systems (9). In elderly individuals, decrease in interleukin-15 (IL-15) increases markers of growth stop indicator and decreases KGF and fibroblast growth factors (FGF) (28). Prolonged inflammation in aged and obese individuals increases pro-inflammatory chemokines and cytokines in the wound area, which impedes wound healing (29).
Chronic stress slows wound repair by increasing circulatory glucocorticoid hormone because it reduces migration of inflammatory cells and pro-inflammatory cytokines to the wound and damages anti-bacterial function, that finally slow healing. Delayed wound repair in depressed and nervous people is four times more than in healthy ones (1). Psychological stress delays wound healing via downregulation of the immune system (9). Stress increases inflammatory cells and decreases angiogenesis and differentiation of the fibroblast to myofibroblast and low matrix deposition in the wound (30). Another mechanism through which stress delays wound healing is the reduction in collagen synthesis (26).
Prolonged hypoxia, as a result of insufficient perfusion and low angiogenesis, is harmful in wound healing. Hypoxia potentiates inflammation and increases ROS (9). In diabetes, decrease in angiogenesis and production of growth factors, abnormal inflammatory and immune response, prolonged inflammatory phase, decreased contraction of the wound, and imbalance between construction and decomposition of extracellular matrix and their remodeling delay wound healing (31).
4.7.3. Positive Factors on Wound Healing
Much research has been done on wound healing, and various materials have been prepared and introduced to accelerate it. Natural substances like N. sativa, honey, and green tea extract have been shown to shorten wound healing time due to stimulation of angiogenesis, improvement of fibroblast proliferation, collagen synthesis, and reduction of inflammation (5).
Grapeseed oil interferes with cell proliferation and angiogenesis due to its antioxidant and anti-inflammatory properties (9). Another beneficial natural substance in wound healing is olive oil. According to a study, olive oil speeds up wound healing because it contains essential fatty acids (oleic acid and linoleic acid), antioxidant, antimicrobial, and anti-inflammatory properties (32). A study showed that Gazangabin extract had no effect on wound contraction time, but it reduced the number of neutrophils, increased the number of eosinophils, and reduced the severity of inflammation (33). Topical application of honey increases granulation tissues depth, accelerates angiogenesis, elevates fibroblast density, and subsidence inflammation in the wound bed (34). Thus, the healing property of honey is due to its antimicrobial and anti-inflammatory properties, moisturizing the wound bed, osmotic effects, diminishing oedema, quickening angiogenesis and granulation tissue formation, accelerating epithelialization, increasing activities of lymphocytes and phagocytes, debridement of necrotic tissue (15), low pH, hydrogen peroxide content, absorption of excessive wound exudate, antibacterial effects, and anti-inflammatory properties (10). Fibroblasts require an acidic environment for activation, and low pH of honey provides optimal conditions. Lysozyme enzyme of honey has a potent antimicrobial property and stimulates or inhibits the release of cytokines from macrophages (35). Topical application of sodium phenytoin ointment 1% increases fibroblasts, granulation tissue formation, and collagen production in the bed of the wound (36).
Nowadays, exercise is recommended as an inexpensive and safe way in the prevention and treatment of chronic wounds (4).
4.7.4. The Effects of Exercise on Wound Healing
Exercise, as complementary medicine, can be used as an intervention strategy to accelerate wound healing (6). It has a positive effect on the immune system, endocrine function, and psychological problems such as stress (1). A clinical study indicated that high-level physical activity after surgery enhanced surgical wound healing (37). Even one session of exercise increases the number of circulatory endothelial progenitor and angiogenic cells that improves vascular synthesis (18).
Many studies have shown that exercise has an important role in the healing of any type of wound. For example, one study reported that exercise promotes oral mucosal wound healing, and another study showed that Kegel exercise in the first week of post-partum accelerated perineum wound healing (38). Exercise is even effective in treating venous leg ulcers (39).
The lively legs (a programmed walking and leg exercise) accelerated wound closure (40). Hence, exercise has a positive effect on the homeostasis phase through the increasing activity of platelets and coagulative factors (15).
4.7.5. Effect of Exercise on Inflammation in Wound
Exercise reduces systemic and local inflammation in obese and aged individuals and significantly reduces the duration of the inflammatory phase in aged mice (6). Reduction of inflammation as a result of exercise is obtained with a decrease in visceral fat mass, decrease in the Toll-like receptors, and increase in anti-inflammatory cytokines (24). Regular moderate exercise prevents oxidative and nitrosative stress by potentiating antioxidant defense/repair systems (41).
Many epidemiological studies demonstrated that regular exercise decreased inflammatory markers (4). As a result of the anti-inflammatory property of regular exercise, inflammatory cytokine level was lower in exercised aged mice in comparison with sedentary ones (4). Also, 12 weeks of moderate swimming exercise after tooth extraction increased the number of polymorphonuclear neutrophils (PMNs) and macrophages in the wound (4). The greatest effect of exercise was seen 1 - 5 days post-wounding, especially on the first day. The studies showed that doing exercise was beneficial on the first day after the injury, especially in the inflammatory phase (4).
Regular exercise reduces C-reactive protein (CRP) levels and suppresses systemic low-grade inflammation. In the wound of mice that exercised, inflammatory cytokines levels were low (4). Contracted skeletal muscle is an endocrine organ and releases myokines that may mediate the beneficial effects of exercise on wound healing. IL-6 is the first cytokine that raises up to 100-fold in circulation during exercise and inhibits the production of TNF-α and IL-1. The increase of IL-6 is dependent on the duration and intensity of exercise and muscle mass that is involved in the training. IL-6 is an anti-inflammatory and immunosuppressive cytokine (42). IL-15 is an essential mitochondrial signal that helps in wound closure. Its effect is mediated by reducing the growth arrest factor and increasing keratinocyte and fibroblast growth. The positive effect of exercise on wound healing in the elderly is mediated by circulating IL-15 (28). In aged mice, exercise increases circulatory IL-15, which initiates signal transducer and activator of transcription 3 (STAT3) signaling pathway, reduces growth arrest factor, and increases keratinocyte and fibroblasts (28). Also, 12 weeks of moderate exercise reduced pro-inflammatory cytokines. There is a reverse relationship between the level of physical activity and inflammation. CRP levels of active individuals are less than their sedentary counterparts. The anti-inflammatory effect of exercise depends on the age, the length and intensity of exercise, and the previous subject’s fitness level. It seems that the effect of exercise on inflammation depends on its length and intensity (4), because they are important factors in the regulation of pro-inflammatory molecules concentration (19).
4.7.6. Effect of Exercise on Oxidative Stress in Wound
Much evidence supports the positive role of moderate aerobic exercise in the reduction of oxidative stress by increasing antioxidant enzyme activities (4). Exercise increases the production of ROS that supports vascular growth and increases tissue vascularization (16). Overproduction of ROS during the exercise augments inflammation which stimulates angiogenesis (18). It has been demonstrated that exercise can speed up wound healing in diabetic and aged individuals (43), because in this pathological situation overproduction of ROS results in delayed healing (4). Evidence indicated that exercise can prevent the damage of ROS by increasing antioxidant enzymes activities. Hoffman-Goetz et al. (cited in Keylock and Young [2010]) reported that 16 weeks of exercise increased the expression of catalase and glutathione peroxidase in mice. One year of training increased glutathione peroxidase and superoxide dismutase in rats, and six months of aerobic exercise increased resting levels of glutathione peroxidase and superoxide dismutase. It seems that regular exercise can prevent oxidative stress and accelerate healing by potentiating the body’s systemic antioxidative defense (4).
4.7.7. Effect of Exercise on Blood Supply and Angiogenesis in Wound
Oxygen is important in the synthesis of connective tissue and the prevention of wound infection. Exercise provides adequate oxygen supply to wound tissue and helps in healing (37). In patients with venous leg ulcers, 12-week exercise intervention as an adjunctive treatment to standard care significantly accelerated ulcer healing because, during walking, the calf muscles act as pumps and improve blood circulation (44). Ankle exercise in diabetes has a positive effect on lower limb wound healing by rising blood (45). In a study, 1 - 5 days of exercise after injury reduced pro-inflammatory chemokines and TNF-α in the wound of aged mice. The possible mechanisms may be an increase in oxygen partial pressure and blood supply (46). PMNs and macrophages in the wound area need oxygen to digest microorganisms and necrotic tissue (45). Moderate intensity exercise by improvement in tissue oxygenation accelerates the wound healing process (4).
One problem in the refractory wound is inadequate blood supply in the margin of the wound. Regular low-intensity endurance exercise accelerates wound healing by improving vascular regeneration and local blood supply in the wound area through elevating endothelial progenitor cells (EPCs) and circulatory vasoactive factors in peripheral blood. An increase in the number of circulating EPCs in the peripheral blood and secretion of vasoactive factors are two main indicators in the capacity of low-intensity exercise in promoting wound healing (12).
Moderate exercise three days after tooth extraction significantly increased expression of VEGF. Regular exercise increases adrenaline, which stimulate the expression of VEGF in macrophages (24). VEGF and nitric oxide (NO) are necessary for angiogenesis. Four weeks of endurance training can increase capillary network (16). Furthermore, exercise increases NO production that is upstream of VEGF (18).
4.7.8. The Effect of Intensity and Type of Exercise on Wound Healing
The studies evaluating the effect of exercise on wound healing have used different training protocols. While some studies used high intensity, others used moderate intensity, which mimic the recommended volume of exercise for the general population to promote health. Based on the results of reviewed studies, moderate-intensity training leads to better wound closure compared to high-intensity and strenuous-intensity (19). Heinen et al. reported moderate-intensity exercise as the best exercise in wound healing in diabetic patients (40). The strong evidence indicated that regular moderate-intensity exercise activates several signaling pathways and produces an anti-inflammatory and antioxidant response (47). Strenuous exercise causes inflammatory reactions due to the overproduction of free radicals (19), and increases TNF-α and IL-6 levels in serum that attract inflammatory cells in the wound bed and delay healing (30). The numbers of PMNs and macrophages, as indicators of wound healing in aerobic exercises, were more than anaerobic exercises, and in both of them were more than sedentary ones. Both aerobic and anaerobic exercises speed up wound healing, although aerobic exercise is better than anaerobic exercises. Anaerobic exercises increase ROS production in wound bed that disturbs healing (17).
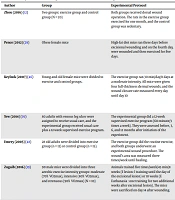
Pence et al. reported that short-term treadmill exercise (three days before and five days after wounding) had no positive effects on the speed of wound healing in lean rats, but it accelerated wound healing in obese ones. They concluded that the positive effects of exercise depend on the inhibition of the expression of pro-inflammatory cytokines such as IL-1 and TNF-α (29). Emery et al. showed that three months of moderate-intensity aerobic exercise accelerated wound healing in older adults (48). However, short-term exercise had no positive effect on wound healing (1). The characteristics of exercise like length, frequency, duration, and intensity are summarized in Table 1.
| Author | Group | Experimental Protocol | Results |
|---|---|---|---|
| Zhou et al. (2016) (12) | Two groups: exercise group and control group (N = 20) | Both groups received dorsal wound operation. The rats in the exercise group exercised for one month, and the control group was sedentary. | The wound size in the exercise group was smaller than in the control group. The exercise group had many circulating EPCs and vasoactive factors more than the non-exercise group. Low-intensity exercise accelerated wound healing. |
| Pence et al. (2012) (29) | Obese female mice | High-fat diet mice ran three days before excisional wounding and on the fourth day, were wounded and then exercised for five days. | Obesity impaired wound healing (P < 0.05). Exercise did not affect wound healing in lean mice. The wound area was smaller in exercised obese mice compared with controls (P < 0.05). There was no difference in gene or protein expression of pro-inflammatory cytokines. |
| Keylock et al. (2007) (46) | Young and old female mice were divided to exercise and control groups. | The exercise group ran 30 min/day/8 days at a moderate intensity. All mice were given four full-thickness dermal wounds, and the wound closure rate measured every day until day 10 | Wound size was reduced significantly in exercised young mice (P = 0.10). In the old mice, exercise significantly decreased wound size (P < 0.05). Inflammatory markers were significantly lower in the wounds of old mice (P < 0.05). Exercise accelerated wound healing in old mice due to its anti-inflammatory effect. |
| Tew et al. (2015) (39) | 80 adults with venous leg ulcer were assigned to receive usual care, and the experimental group received usual care plus a 12-week supervised exercise program. | The experimental group did a 12-week supervised exercise program (60-minute/3 times a week). They were assessed before, 3, 6, and 12 months after initiation of the experiment. | Exercise, as complementary medicine, was beneficial in the acceleration of leg ulcer healing. |
| Emery et al. (2005) (48) | 28 old adults were divided into exercise group (n = 13) or control group (n = 15). | The exercise group did the routine exercise, and both groups underwent an experimental wound procedure. The wound’s area was measured three times/week until healing. | A relatively short-term exercise intervention was associated with an enhanced rate of wound healing in healthy elderly. |
| Zogaib and Monte-Alto-Costa (2011) (19) | 90 male mice were divided into three aerobic exercise intensity groups: moderate (70% VO2max), intensive (80% VO2max), and strenuous (90% VO2max) (N = 10) | Animals trained five times/week/45 min/8 weeks (E lesion: l training until the day of the excisional lesion) or 10 weeks (E Euthanasia : one training for two additional weeks after excisional lesion). The mice were sacrificed on day 14 after wounding. | Moderate-intensity exercise led to faster wound closure than controls and M/E euthan (P < 0.05). Moderate-intensity training showed better re-epithelialization than controls (M/E lesion = 85.9%, M/E euthan = 96.4% and M/CG = 79.9%; P < 0.05). Moderate-intensity exercise was a good method in accelerating skin wound healing. |
| Wolfe (2013) (8) | Female diabetic mice were divided into three groups of control, low-intensity exercise, and high-intensity exercise | Mice exercised for 30 minutes/five days/week for three weeks with low intensity or high intensity. Three days after initiation of exercise, the mice were wounded. | Low-intensity exercise improved the healing of wounds better than high-intensity exercise. |
| Irmawati et al. (2018) (24) | Rats were divided into control and exercise group | The exercise was a moderate exercise with 50% maximal work capacity every day for two weeks. The VEGF expression was assayed three days after tooth extraction. | The exercise group had a higher expression of mean VEGF as compared to the control group. Moderate exercise increased the expression of VEGF during the wound. |
| O'Brien et al. (2017) (44) | 63 patients were randomized to receive either a 12-week exercise in addition to routine care intervention or usual care | 12-week exercise intervention in conjunction with usual care | 70% of the intervention group and 53% control group were healed. The participants who exercised healed better than sedentary ones (P = 0•045). |
| Monte‐Alto‐Costa (2015) (26) | Mice were divided into three groups: control, stressed, and stressed‐exercised | The exercise protocol was moderate intensity for eight weeks. After six weeks of initiation of training, the stressed group was submitted to stress till the end of the experiment. On week eight, all mice were wounded, and then exercise stopped. | In the sedentary group, higher inflammatory cells and a thinner neo‐epidermis were seen. In the exercised group, many myofibroblasts and blood vessels were seen. In the stressed group, less and immature collagen fibers were seen. In stressed animals exercise impaired wound closure. |
Summary of Studies Evaluating the Effect of Execrise on Wound Healing
5. Conclusions
According to the results of reviewed studies, wound healing consists of four phases, and disturbance in each stage leads to a delay in wound healing. In some situations, like ageing, obesity, stress, and diabetes, a chronic low-level inflammation as a result of the increase in oxidative stress increases inflammatory phase duration that impairs skin regeneration. Substances like honey or green tea with reduced inflammation help in shortening wound healing length. It is well-known that regular moderate-intensity exercise has an anti-inflammatory effect through potentiating the antioxidative system, increasing systemic levels of anti-inflammatory cytokines (e.g., IL-6), inhibiting the production of inflammatory cytokines, and reducing resting cortisol levels. In addition to the anti-inflammatory effects, regular exercise improves angiogenesis and increases local blood flow that provides oxygen and nutrients to wound tissue, which is important in the synthesis of connective tissue and the prevention of wound infection. Exercise potentiates the turnover of collagen in connective tissue and strengthens scars. While regular exercise accelerated the healing process, short-term exercise had no positive effects on it. Strenuous exercise causes an inflammatory reaction that increases inflammatory cells and delays healing. Moderate-intensity exercise as a low-cost intervention is a beneficial strategy in the treatment of impaired and chronic wounds and shortening healing duration, and it can be recommended as complementary medicine in the clinic to accelerate wound healing.