1. Background
One of the most important medical discoveries of the 20th century is the discovery of penicillin by Fleming in 1929. Although penicillin significantly reduced mortality and morbidity due to common infections, antibiotic-resistant bacteria were quickly observed in laboratories and shortly after (1) in clinical medicine (2).
The excessive use of antibiotics such as penicillin (G), which are among the most widely used antibiotics in Iran, has caused them to appear in the wastewater entering refineries upon defecation from the host’s body (3), causing many problems in the environment. High concentrations of antibiotics in the wastewater produced by antibiotic production companies and hospitals can interfere with the biological refinement of wastewater. On the other hand, these pollutants appear in groundwater resources by the penetration of hospital wastewater and pharmaceutical wastes into these reservoirs and are not generally removed through basic refinery processes such as sedimentation pools and chlorination due to their solubility in water. With the growing population in large cities, there has also been an increase in drinking water contamination (4, 5).
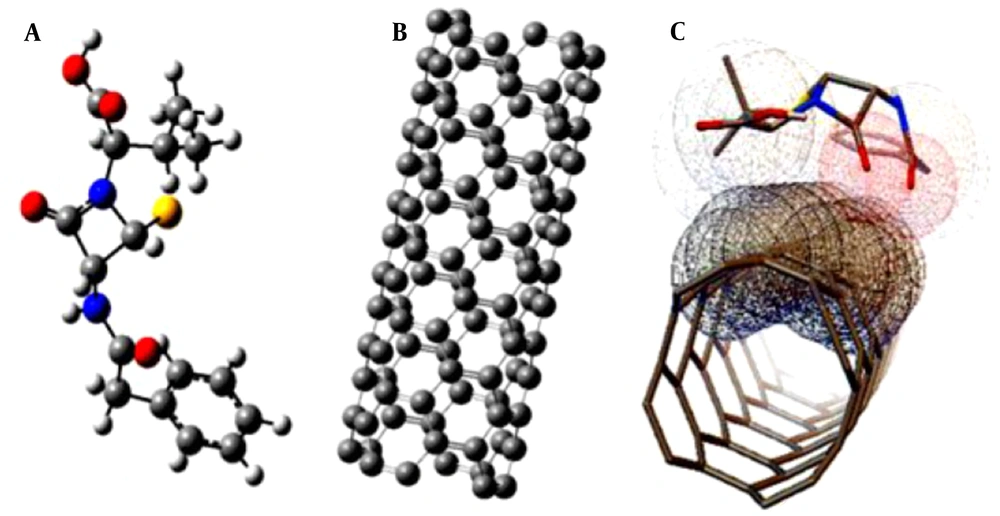
Therefore, it seems necessary to conduct research on penicillin (G) detection. In this regard, single-layered nanotubes are among the nano-devices that significantly influence the sensitivity of biosensors. Besides, carbon nanotubes (CN), because of their unique features, are commonly used as nano-sensors. Sensors are capable of detecting and altering the measurable physical properties that are generated by electrical signals (6). Accordingly, we aimed to investigate the interaction of penicillin (G) with CN using computational chemistry and molecular docking. By evaluating thermodynamic parameters and the amount of heat generated by penicillin (G) absorption based on these calculations, we checked if this nanostructure can be used in designing penicillin (G) biosensors. For this purpose, Gauss View software was used to design a carbon nanotube penicillin (G) sensor (4). To bring the systems close to the minimum level of energy, the systems were first optimized geometrically using Gaussian software applying the HF technique (the 6-31G basis set). The physical properties of the systems were assessed after calculations by the Auto Dock Tools software, and their stability, electrical changes, and physicochemical properties were calculated and compared. Finally, the most stable interactional states between the nanotubes and penicillin (G) were detected (7, 8) (Figure 1).
2. Methods
First, the structures of penicillin and CN were drawn using nanotube modeler 1.3.0.3 and Gauss view 5.0 software (9). Next, geometric optimization was performed using the density function theory method at B3LYP / 6-31G (d) computational level based on the positive results of previous studies. The Gaussian software 10 was used to perform calculations at the temperature of 298 K and a pressure of 1 atmosphere. Afterwards, Auto Dock Tools software (Auto Dock, version 4.2) was used (10). A total of ten conformation models were generated using the Auto Dock, indicating best models to predict the interaction between the antibiotic and the CN. In addition, a series of energy values (binding energy, ligand efficiency; inhibition constant; intermolecular energy; Van der Waals, electrostatic, and total internal energy) were generated using the Lamarckian program (Trott and Olson, 2009) (10).
3. Results and Discussion
3.1. Structural Properties
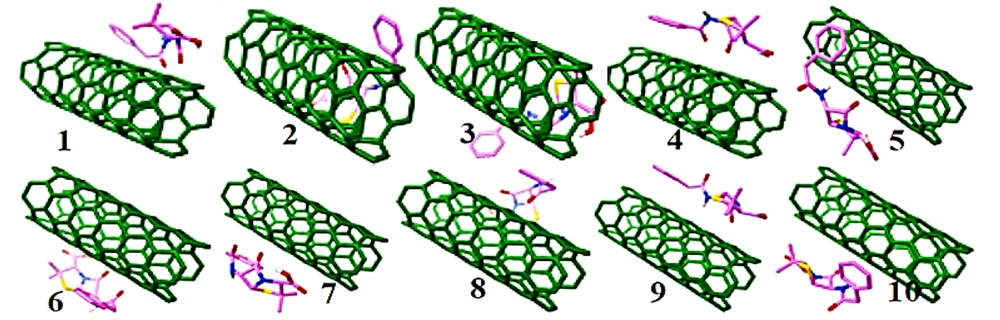
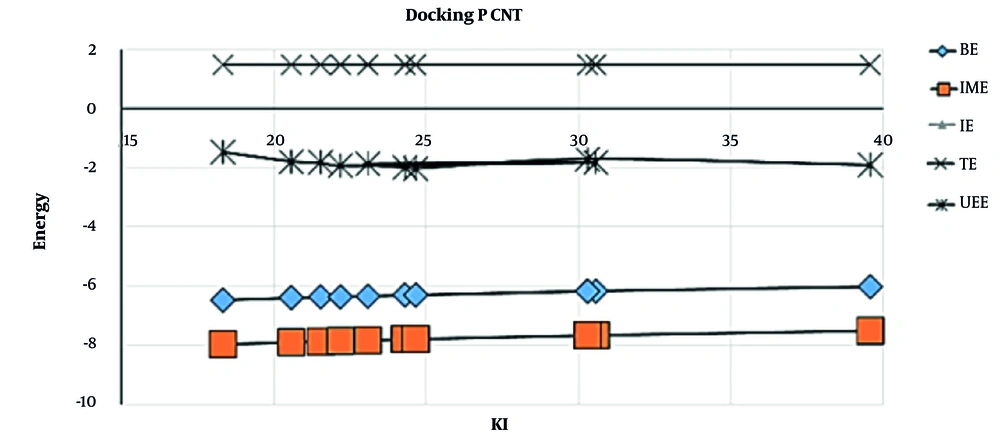
The docking method and Auto Dock software were used to select the most stable configuration of penicillin (G) with nanotubes (Figure 2). This figure is according to the states ranking based on the coherence energy (Table 1).
| Results of Docking for P CNT | |||||
|---|---|---|---|---|---|
| KI | BE | IME | IE | TE | |
| 1 | 18.33 | -6.46 | -7.95 | -1.47 | 1.49 |
| 2 | 20.58 | -6.39 | -7.89 | -1.78 | 1.49 |
| 3 | 21.53 | -6.37 | -7.86 | -1.82 | 1.49 |
| 4 | 22.19 | -6.35 | -7.84 | -1.93 | 1.49 |
| 5 | 30.57 | -6.16 | -7.65 | -1.82 | 1.49 |
| 6 | 23.09 | -6.33 | -7.82 | -1.86 | 1.49 |
| 7 | 24.30 | -6.30 | -7.79 | -1.99 | 1.49 |
| 8 | 24.66 | -6.29 | -7.78 | -2.02 | 1.49 |
| 9 | 30.29 | -6.16 | -7.66 | -1.70 | 1.49 |
| 10 | 39.58 | -6.01 | -7.50 | -1.90 | 1.49 |
Abbreviations: BE, binding energy; IME, intermolecular energy; IE, internal energy; TE, torsional energy; kI, inhibition constant.
The energy changes were negative for penicillin (G) derivatives with carbon nanotube (Table 1 and Figure 3), indicating the exothermic value of the adsorption process. Hence, it can be argued that physical adsorption of penicillin (G) can interact with CN, indicating its potential to be administered in the construction of new thermal sensors intended to measure penicillin (G) (11). Temperature measurement process of theses sensors is based on a highly accurate and sensitive thermistor, which can be administered to evaluate the amount of analytes (12).
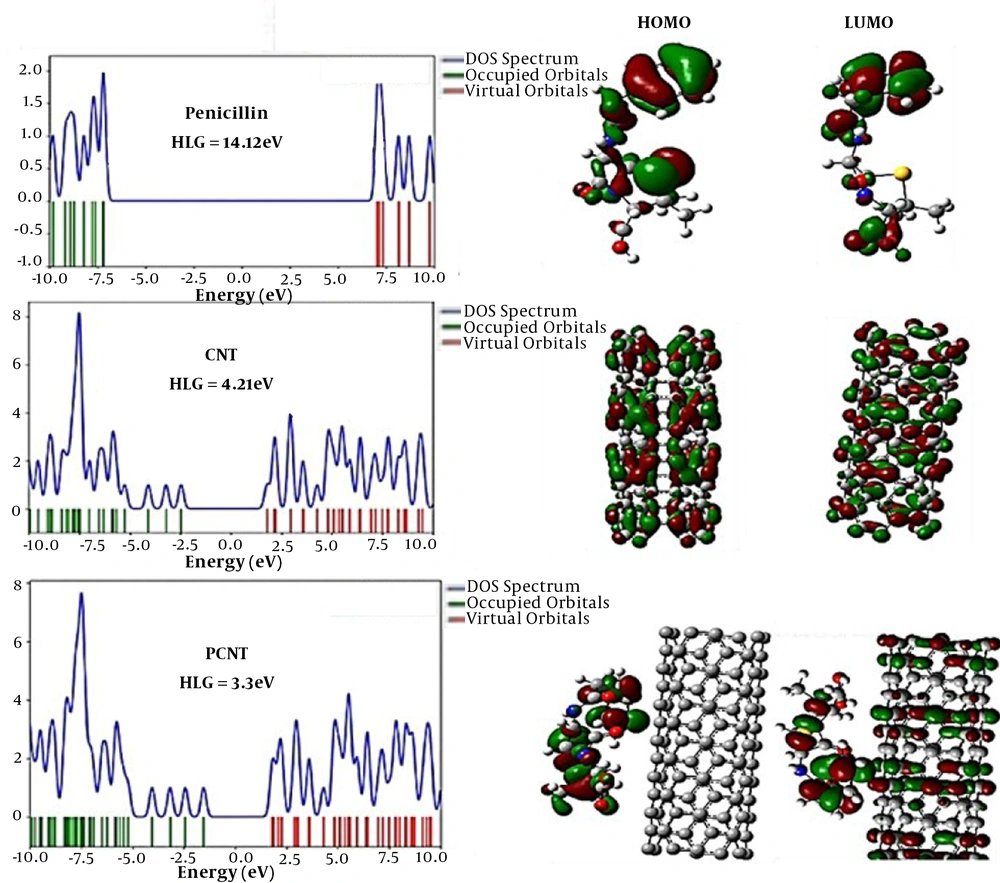
3.2. Analyzing Molecular Orbitals Calculations
According to chemistry literature, HOMO and LUMO are the highest and lowest empty or unoccupied molecular orbital, respectively. The energy gap is the energy difference between the two orbitals, often denoted by the HLG symbol. Equation 2 is developed to calculate the energy gap, where EH and EL indicate the energies of Homo and Lomo orbitals, respectively. The electrical conductivity of the molecules directly affects the energy gap (1). The lower the energy gap, the more efficiently the electron pass from the forbidden band and into the conduction band (13). Hence, materials with lower energy gaps have higher electrical conductivity in comparison to those with higher energy gaps.
As shown in Table 2, following the penicillin (G) absorption on the carbon nanotube’s surface, the energy gap declined. In other words, following interaction with CN, both conductivity and conductivity of penicillin (G) considerably increased. This enhanced conductivity can be employed to identify and measure CN, indicating the potential of CN to be used for the construction of new electrochemical sensors to measure penicillin (G) (14).
| EH (eV) | EL (eV) | HLG (eV) | µ (eV) | η (eV) | S (eV) | ω (eV) | ΔNmax (eV) | Dipole Moment (Debye) | |
|---|---|---|---|---|---|---|---|---|---|
| Penicillin (G) | -7.13 | 6.99 | 14.12 | -0.07 | 7.06 | 0.14 | 0 | 0.01 | 2.74 |
| CNT | -2.43 | 1.78 | 4.21 | -0.32 | 2.11 | 0.47 | 0.02 | 0.15 | 0.0095 |
| P-CNT | -1.52 | 1.78 | 3.3 | 0.13 | 1.65 | 0.16 | 0.01 | -0.08 | 10.96 |
Abbreviations: EH, EHOMO; EL, ELUMO; HLG, gap energy; η, hardness; µ, chemical potential; S, softness; ω, electrophilicity.
The other important parameter is chemical hardness (η), which can be obtained using Equation 3. It is an appropriate parameter to evaluate the reactivity of a new compound, mainly due to the fact that softer molecules with lower chemical hardness can alter their electron density. Hence, soft compounds have the advantage of better and easier electron transfer, which is vital for chemical reactions. Table 2 shows the enhanced adsorption on CN following reactivity of penicillin (G), as all derivatives produced using interaction with CN have lower chemical hardness compared to pure penicillin (G).
Chemical potential (µ) was calculated using Equation 4 and used for calculations related to other parameters. For each compound, Electrophilicity (ω) (Equations 5) and maximum charge transferred to the system (∆Nmax) (Equations 6) show the tendency to absorb electrons. Each molecule reaction contains an electrophilic and a nucleophile. The receptor electron is the compound with higher electrophilicity and charge capacity, i.e., receiving electrons from the system (15). As its electrophilicity is zero electron volts, penicillin (G) contributes to an electron donor in interaction with the nanostructure (Table 2). Meanwhile, pure CN contributes to electron acceptor as its electrophilicity is 0.01 electron volts (6). Hence, using penicillin (G), CN can enter electrochemical reactions18. Moreover, following adsorption on the surface of the carbon nanotube, the electrophilicity of penicillin (G) enhanced (Table 2). Therefore, it enhanced the tendency of penicillin (G) towards electron adsorption (16).
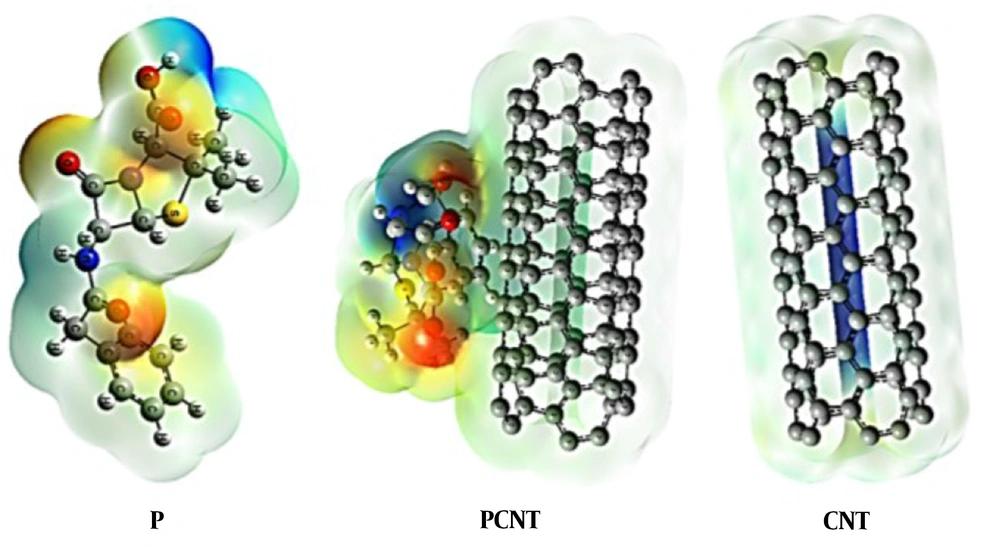
We also investigated the bipolar moment of the structures, which is an appropriate criterion for assessing molecules' solubility in polar solvents (17). Molecules with higher dipole moments show better solubility in water and vice versa (18). According to the findings, following adsorption on the surface of pure CN, the dipole moment of penicillin (G) increased (9). Hence, CN derivatives with penicillin (G) are more water-soluble in comparison to the penicillin (G) (19) (Table 2 and Figure 4).
Electrostatic potential maps are highly beneficial three-dimensional diagrams of molecules because they allow to calculate the charge distributions of molecules and charge-related characteristics of molecules. Furthermore, they can be used to visualize both the size and shape of molecules. Electrostatic potential maps are highly beneficial to predict the behavior of complicated molecules in chemistry. A color spectrum with red (negative charges) and blue (as positive charges), as the lowest and highest electrostatic 10 potential energy value, respectively, was used to convey various intensities of the electrostatic potential energy values. The former shows the least electrostatic potential (loose or excess bound of electrons) and acts as an electrophilic attack, while the latter shows the opposite (Figure 5).
Map of a molecule or electrostatic potential (ESP) surface illustrates the partial distribution of change along the molecule’s surface. These maps are important because they help describe molecular polarity and place the dipole arrow. The ESP surface will be produced, red areas indicating negative poles and blue areas indicating positive poles.
Comparing the results of the present study (molecular docking of penicillin (G) antibiotic with carbon nanotubes) with the results of some previous studies (molecular docking of penicillin with boron nitride nanotubes) shows that both carbon nanotubes and boron nitride with penicillin have exothermic and spontaneous interactions, but this interaction with carbon nanotubes is more effective; this claim is proven by the release of more heat (20).
4. Conclusions
We investigated the adsorption of penicillin (G) on the surface of CN based on the theory of density function and molecular docking. According to the obtained binding energy (calculated at room temperature), the adsorption process was both exothermic and spontaneous. Analysis of molecular orbitals also showed that complex CN with penicillin (G) was more electrophilic, conductive, and reactive than pure penicillin (G). In addition, it showed the potential of CN to develop new electrochemical sensors to identify and evaluate penicillin (G). Structurally, CNs are highly similar to penicillin (G). Further studies are recommended to investigate the potential of CN to remove and measure penicillin (G) and the impact of investigated nanostructures on their energy characteristics.