1. Background
H2O2 plays an essential mediator in several biological reactions catalyzed by enzymes (1, 2). The excess of H2O2 may potentially damage carbohydrates, lipids, and proteins in the human body (3). Thus, it is crucial to design an efficient platform for H2O2 measurement in biological samples. So far, different determination schemes have been used for H2O2 monitoring, such as chromatography (4), spectrophotometry (5), chemiluminescence (6), and electrochemistry (7).
Enzyme-less H2O2 electrochemical sensors have the advantage of simplicity, inexpensive, high sensitivity, rapid response and suitability for real-time detection (8, 9). From this point of view, the construction of new and effective electrochemical assays for H2O2 detection, especially in biological samples, has received extensive attention in recent years (10, 11).
2. Objectives
In this work, MnO2 nanoflakes were deposited on the surface fibrous structure of sepiolite clay via a facile hydrothermal process. The prepared nanocomposite (MnO2/sepiolite) was employed for the modification of a simple and low-cost carbon paste electrode (CPE). The electrocatalytic activity of the modified CPE toward H2O2 was explored. The linear detection range and detection limit of the MnO2/sepiolite-CPE were also investigated in detail. Furthermore, the fabricated non-enzymatic H2O2 electrochemical sensor was used for the determination of H2O2 in human serum samples.
3. Methods
3.1. Reagents and Instrument
Flake graphite (100 mesh, 99.5% purity), paraffin oil, H2O2 (30 wt%), ammonium persulfate ((NH4)2S2O8, 99.0 purity), potassium permanganate (KMnO4, 99.5 purity), disodium hydrogen phosphate dodecahydrate (Na2HPO4 12H2O, 99 purity), and sodium dihydrogen phosphate dehydrate (NaH2PO4 2H2O, 99 purity) were acquired from Merck Co. (Darmstadt, Germany). Sepiolite powder was provided by Dorkav Minig Co., Ltd. (Mashhad, Iran). Raw sepiolite was purified according to a previously reported method (12). Ultrapure water was used for the preparation of phosphate buffer solution.
Electrochemical experiments were conducted on a OrigaState100 electrochemical workstation (OrigaLys, France) using a standard electrochemical cell, including the modified CPE as the working electrode, the platinum wire as the counter electrode, and saturated calomel electrode (SCE) as the reference electrode.
3.2. Synthesis of MnO2/Sepiolite Nanocomposite
MnO2/sepiolite nanocomposite was prepared via the one-step hydrothermal method, described in an earlier report (13). Briefly, 2.0 g of the purified sepiolite powder was dispersed into a 30-mL mixed solution of (NH4)2S2O8 (2.21 g) and KMnO4 (1.85 g). This mixture was poured into a 50-mL Tefon-lined stainless steel autoclave and then kept in an oven at 110°C for 14 h. The achieved MnO2/sepiolite material was collected and then washed repeatedly with ultrapure water. The product was further dried in an oven at 60°C.
3.3. Electrode Fabrication
The working CPE electrode was prepared according to the methods reported previously (14, 15). Typically, materials, including flake graphite, paraffin oil, and MnO2/sepiolite in the ratio of 67:25:8 (w/w), were mixed in a mortar for 10 min to get a homogenized carbon paste. The obtained paste was filled carefully into a Teflon tube (3 mm inner diameter and a height of 10 cm) as the body of the electrode. A copper wire was used as the electrical conductor. A fresh CPE surface was provided with polishing the electrode surface on a weighing paper.
4. Results
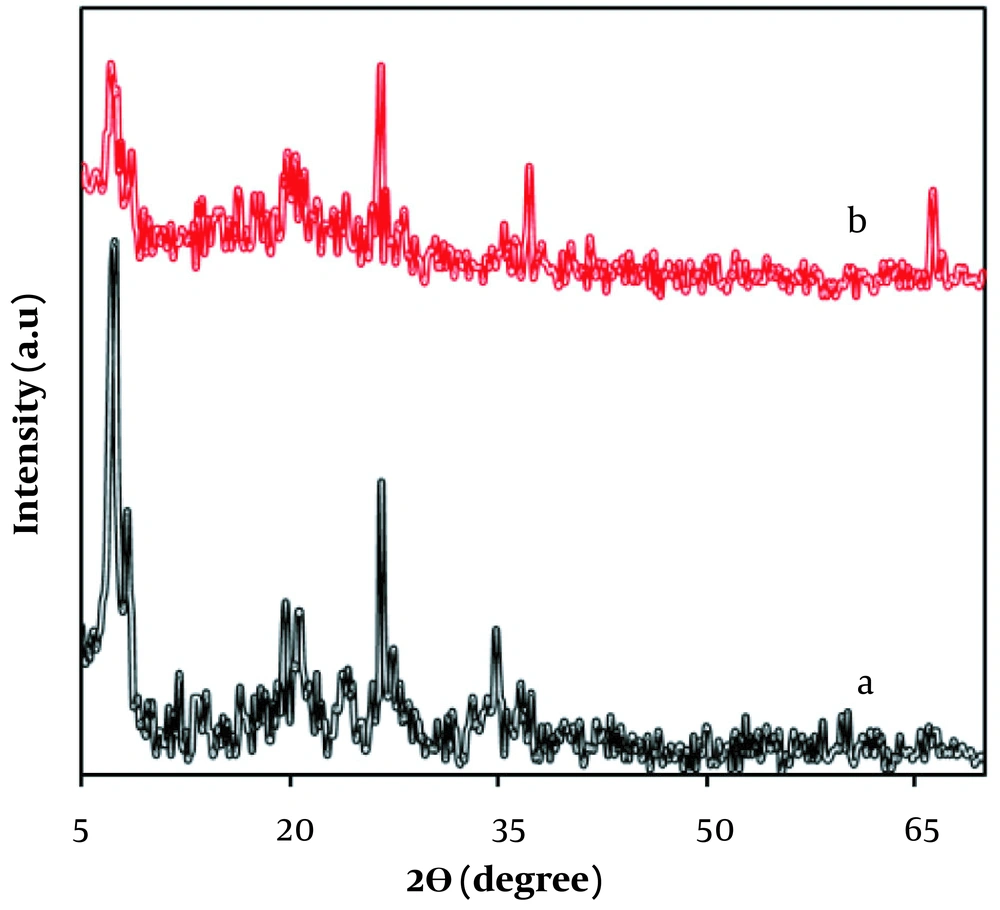
X-ray diffraction patterns of the sepiolite and MnO2/sepiolite samples are shown in Figure 1A and B. The diffraction peaks appeared at 2θ = 7.7°, 19.6°, 20.7°, 26.5°, and 34.8° matched well with the diffraction peaks of (110), (060), (131), (080), and (441) crystal planes of sepiolite clay standard data (JCPDS card PDF file No. 13-0595) (16, 17). Two characteristic diffraction peaks at 37.2° and 66.3° could also be assigned to the (131), and (421) planes of γ-MnO2 (JCPDS 72-1982), respectively (18).
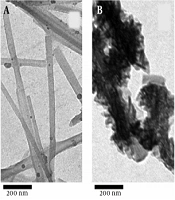
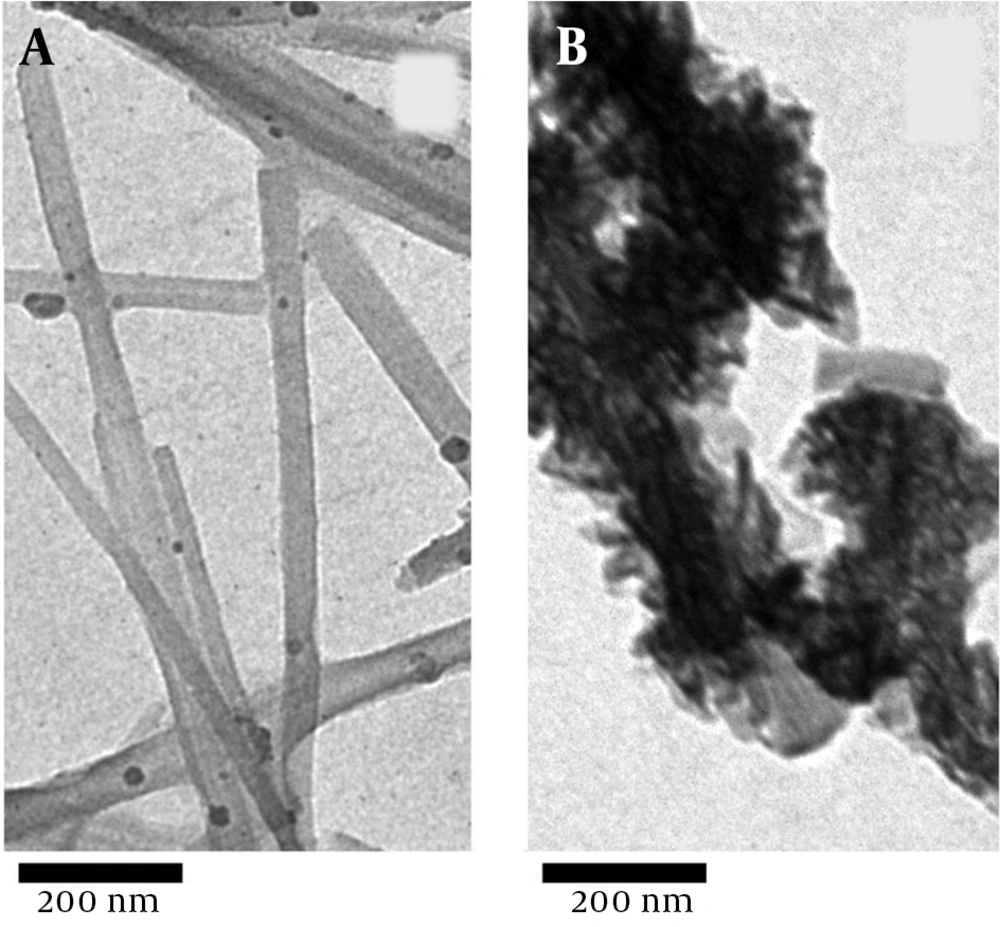
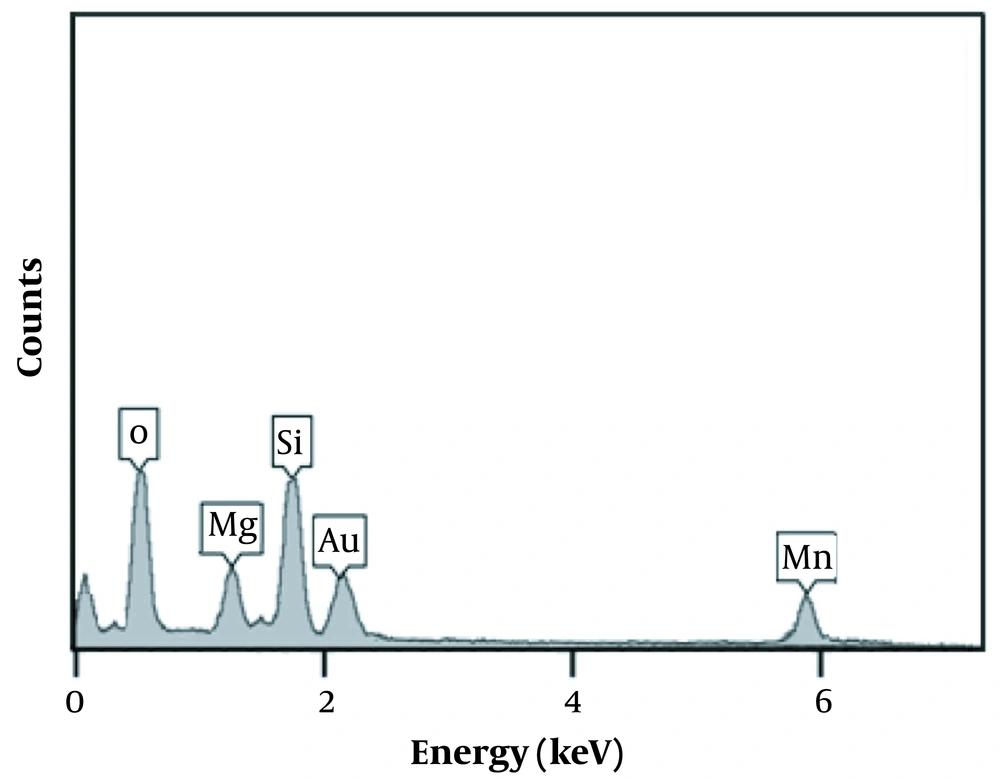
The TEM images of the natural sepiolite and MnO2/sepiolite nanocomposite are shown in Figure 2A and B. As can be seen, MnO2 nanoflakes successfully deposited on the surface of the sepiolite fibers. Moreover, the EDS spectrum of the MnO2/sepiolite nanocomposite (Figure 3) depicts the existence of Mn, O, Si, and Mg and elements in the prepared material. All the above results confirm the synthesis of MnO2/sepiolite nanocomposite via the hydrothermal method.
5. Discussion
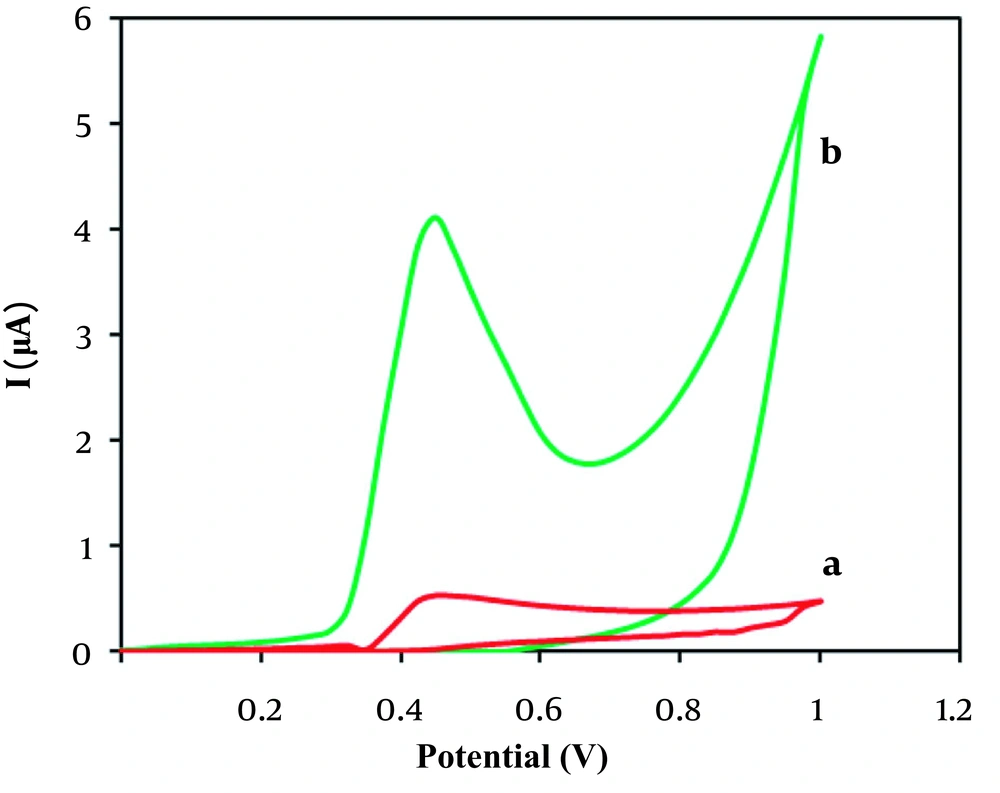
The electrochemical performances of unmodified CPE and MnO2/sepiolite-CPE toward H2O2 were studied by cyclic voltammetry. As presented in Figure 4, the oxidation peak current for MnO2/sepiolite-CPE (appeared at 0.45 V) was much larger than that of the unmodified CPE, which is ascribed to the remarkable catalytic ability of MnO2/sepiolite material toward H2O2 oxidation on the electrode surface.
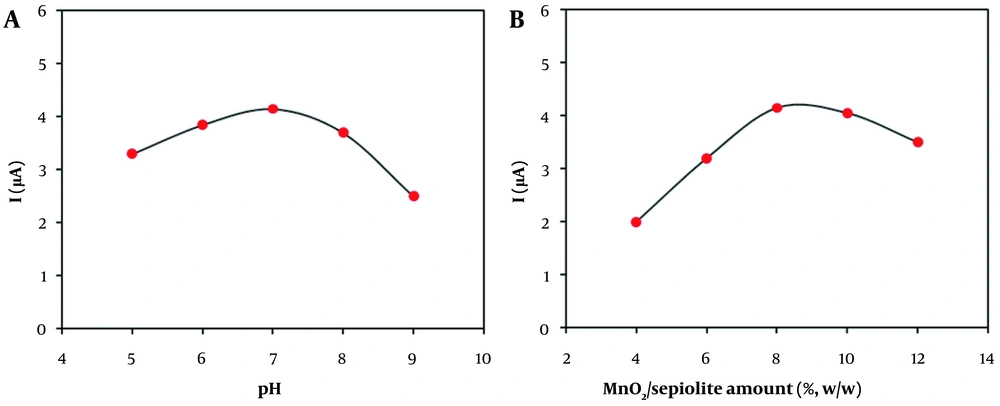
The influence of solution pH was explored on the voltammetric peak current at the MnO2/sepiolite-CPE. As seen in Figure 5A, the voltammetric signals first increased with increasing pH up to 7.0, and then decreased at higher pH values. Thus, the pH 7.0 of phosphate buffer was selected for the following electrochemical tests.
The effect of MnO2/sepiolite dose on the range of 4.0 - 12.0% (w/w) was studied by the voltammetric method in a solution containing 100 µM of H2O2. As shown in Figure 5B, the maximum response can be observed at the amount of 8.0% MnO2/sepiolite. Consequently, it was chosen as the optimal modifier amount in the next experiments.
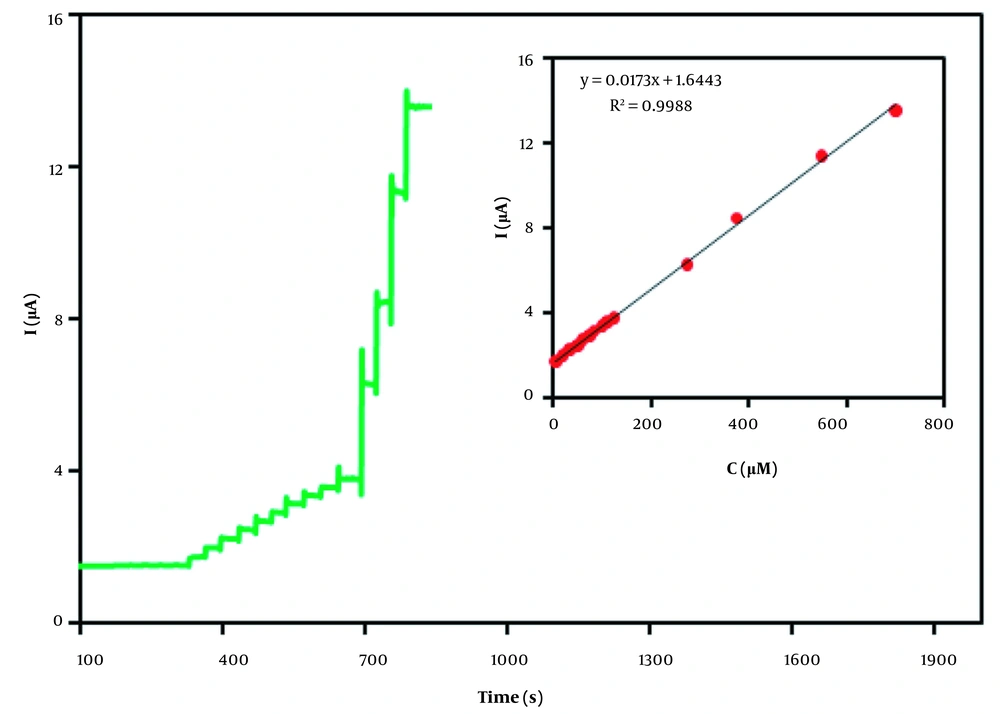
To assess the sensitive response towards H2O2, the current-time (I–t) curve was explored at an applied potential of 0.5 V. The amperometric responses of the MnO2/sepiolite-CPE with the successive injection of H2O2 into 0.1-M buffer solution (pH 7.0) were investigated, and the results are depicted in Figure 6. The linear relationship between amperometric signal current and analyte concentration in the range of 5 - 700 μM could be observed. Furthermore, the limit of detection (based on 3σ) was found to be 0.8 μΜ, which was less than that of other methods (19-24) as listed in Table 1. Besides, the relative standard deviation (RSD) for ten replicate detections of 50 µM H2O2 was calculated as 2.6%. It was also noticed that the MnO2/sepiolite-CPE showed good stability and could be used for at least two weeks. The influence of common interfering species on the determination of 50 µM H2O2 using the MnO2/sepiolite-CPE was evaluated. As listed in Table 2, the 10-fold concentration of interfering molecules demonstrated nearly no interference in H2O2 monitoring. This finding indicated the satisfactory selectivity of the suggested assay.
| Electrode Modifier* | Linear Range (µM) | Detection Limit (µM) | Ref. |
|---|---|---|---|
| MnO2 nanotubes/reduced graphene oxide nanocomposite | 100 - 30000 | 1.29 | (19) |
| V2O5/VO2 nanostructures | 8 - 215 | 5 | (20) |
| Cuprous oxide-reduced graphene oxide nanocomposites | 30 - 12800 | 21.7 | (21) |
| Poly(p-aminobenzene sulfonic acid) | 50 - 550 | 10 | (22) |
| Hematite nanoparticles | 50 - 3145 | 22 | (23) |
| Gold nanobipyramids/multi-walled carbon nanotubes | 5.0 - 47300 | 1.5 | (24) |
| MnO2/sepiolite nanocomposite | 5 - 700 | 0.8 | This work |
| Foreign Molecule | Recovery (%) |
|---|---|
| Sucrose | 97.9 |
| Citric acid | 98.0 |
| Glucose | 97.7 |
| Ascorbic acid | 97.2 |
| Glutamic acid | 98.6 |
The practical applications of the MnO2/sepiolite-CPE in analysis of H2O2 in human serum samples were studied using the standard addition method. Real samples were provided from a local hospital in Tehran. The obtained results and recoveries of the spiked samples are exhibited in Table 3. These results showed that the present system is an effective platform for the monitoring of H2O2 in real applications.
| Sample | Spiked (µM) | Found (µM) | Recovery (%) | RSD a |
|---|---|---|---|---|
| Human serum (1) | 10 | 9.8 | 98.0 | 3.5 |
| 20 | 20.3 | 101.5 | 4.1 | |
| 30 | 29.4 | 102.0 | 3.4 | |
| Human serum (2) | 10 | 10.3 | 103.0 | 3.5 |
| 20 | 19.7 | 98.5 | 4.2 | |
| 30 | 28.9 | 96.3 | 4.0 |
a n = 3.
5.1. Conclusion
In sum, a simple, selective, and sensitive electrochemical device for H2O2 determination was proposed. The MnO2/sepiolite-CPE showed a good linear relationship with the concentration of H2O2 up to 700 µM. Moreover, the suggested method showed notable selectivity for the measuring of H2O2 in the presence of some interfering species. In addition, MnO2/sepiolite-CPE demonstrated great potential application for H2O2 monitoring in real biological samples.