1. Context
Acute respiratory syndrome coronavirus 2 (SARS-CoV-2) appeared in late December 2019 in Wuhan, China, and the disease caused by the virus was named coronavirus disease 2019 (COVID-19). The virus spread rapidly worldwide, creating a deadly pandemic that has continued to this day (1). Iran was one of the first countries which faced the widespread outbreak of COVID-19 and has one of the highest mortality rates regarding the number of cases (2, 3). The disease is asymptomatic or mild in most patients. However, a significant percentage of patients develop severe pneumonia, which can lead to hypoxemic respiratory failure, shock, organ dysfunction, and death (4).
The COVID-19 pandemic is arguably the biggest challenge humans have faced since World War II (5). This pandemic poses a serious threat to public health and economic stability around the world, (6) affecting a vast majority of the world’s population, and the death rate from SARS-CoV-2 is changing daily (7). During the COVID-19 pandemic, individuals worldwide encounter issues, such as global lockdown, anxiety, stress, and major healthcare challenges (8). Since the outbreak of the disease, various countries have reached the conclusion that the accurate, efficient, comprehensive, reliable, and up-to-date assessment of available information plays a vital role in the effective management of this incident (9). Although several therapeutic compounds and drugs have been recommended to overcome COVID-19, they are often considered supportive treatment options (10, 11).
Since January 2020, numerous countries have taken precautionary measures to control the transmission of SARS-CoV-2 in the hope of developing safe and effective vaccines (12). These two main factors might raise concern for some individuals about the possibility of severe side effects after vaccination. However, there have been numerous reports of expected adverse effects (13). Scientists have come to the conclusion that there is no other way to end this pandemic except through public vaccination. Nevertheless, these newly developed vaccines can cause frequent side effects among populations that might decrease quality of life and induce some concerns (14).
2. Objectives
With this background in mind, the current review aimed to evaluate the short-term side effects associated with COVID-19 vaccines, which are more commonly injected in Iran.
3. Methods
The article databases, such as PubMed, Embase, EBSCO, Medine, PubMed Central, and Google Scholar, were used to prepare this review, and 97 English articles and 14 English websites within 2019 - 2021 containing at least one keyword, such as side effects, COVID-19, vaccine, AstraZeneca, Sputnik V, and Sinopharm, were selected and studied. Then, the appropriate studies were reviewed based on the publication date, the name of the first author, and the relevance of the title and its purpose. Finally, 48 English studies and 10 English websites were selected for final analysis and review article writing.
4. Results
The SARS-CoV-2 was first observed in Wuhan, China, in December 2019 and endangered the world’s health in a very short time (15). According to researchers’ statements and the lack of effective antiviral drugs, the development of safe and effective vaccines is the only approach to control the COVID-19 pandemic (16, 17). For the development of vaccines against SARS-CoV-2, different platforms have been considered as follows:
(1) Messenger ribonucleic acid-based vaccines, which are the latest generation of produced vaccines: Moderna and Pfizer (Pfizer-BionTech) vaccines fall in this category (18, 19).
(2) Vaccines containing viral vectors developed by new methods of biotechnology: Oxford-AstraZeneca, Sputnik V, and Johnson & Johnson vaccines are of this type (18, 20). According to studies, these vaccines have shown a good humoral and cellular immune response after the first dose, and the injection of the second dose develops a stronger and longer-lasting immunity (21-23).
(3) Vaccines containing the killed/inactivated virus that include all or part of a nonliving virus, including Sinopharm, Sinovac, and Bharat Biotech vaccines: (18, 24) The inactivated virus vaccine platform has been widely used for over 70 years. In these vaccines, the viruses are neutralized by chemicals, ultraviolet rays, and heat, and finally, a safe vaccine is developed, especially suitable for individuals with a defective immune system. However, they produce lower immune responses than vaccines containing live viruses and require booster doses (25, 26). Vaccines containing viral vectors are considered a preventive approach against the pathogen and cause the long-term expression of antigenic proteins. Therefore, they provide a better preventive effect by activating cytotoxic immune T cells (which lead to the removal of virus-infected cells) (27, 28).
(4) Subunit vaccines containing a piece of the virus: These vaccines do not contain genetic structure and are therefore considered safe. Novavax belongs to this group (19, 29).
In the following section, the side effects of Sinopharm, AstraZeneca, and Sputnik V vaccines, which have been more frequently used in Iran, will be reviewed:
On August 11, 2020, Russia authorized a vaccine against SARS-CoV-2 called “Sputnik V” manufactured by the Gamaleya Institute in Moscow. The difference between the Sputnik V vaccine and other vaccines is the use of two different adenovirus vectors instead of a single serotype, namely adenovirus 5 and adenovirus 26. The advantage of this strategy is the production of antibodies against the adenovirus 26 serotype after the first dose and against the adenovirus 5 serotype after the second dose, which improves the immune response (30-32).
The Oxford-AstraZeneca vaccine (ChAdOx1 nCoV-19 vaccine/AZD1222), which uses a nonreplicating chimpanzee adenovirus as a vector to express SARS-CoV-2 spike, was approved by the World Health Organization (WHO) for injection to individuals over 18 and over 65 years of age on February 15, 2021. The vaccine was developed jointly by the University of Oxford, England, and the AstraZeneca Institute (16, 33). The European Medicines Agency (EMA) has also fully evaluated and approved the quality, safety, and efficacy of this vaccine (34). The Sinopharm COVID-19 vaccine, developed by the Institute of Biological Products in Beijing, was approved by the WHO on May 7, 2021, as the first Chinese vaccine against COVID-19 (35, 36). The information of these three vaccines (ie, AstraZeneca, Sputnik V, and Sinopharm) are available in Table 1.
| Variables | Type | Efficacy | Dose | Storage | Interval of Vaccine |
|---|---|---|---|---|---|
| Sputnik V | Adenovirus-based | 91.6% efficacy against the original strain of the virus; 100% against severe COVID-19 | 2nd | -18.5°C | 21 days apart |
| Sinopharm | Inactivated SARS-CoV-2 (Vero cell) | 79% against symptomatic SARS-CoV-2 infection 14 days or more after the second dose; 100% against severe COVID-19 | 2nd | 2 - 8°C | 21 - 28 days apart |
| AstraZeneca | Adenovirus-based | 76% against symptomatic COVID-19; 100% against severe or critical disease and hospitalization; 85% against symptomatic COVID-19 in individuals of 65 years and older | 2nd | 2 - 8°C | 28 days apart |
Abbreviations: SARS-CoV-2, acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease 2019.
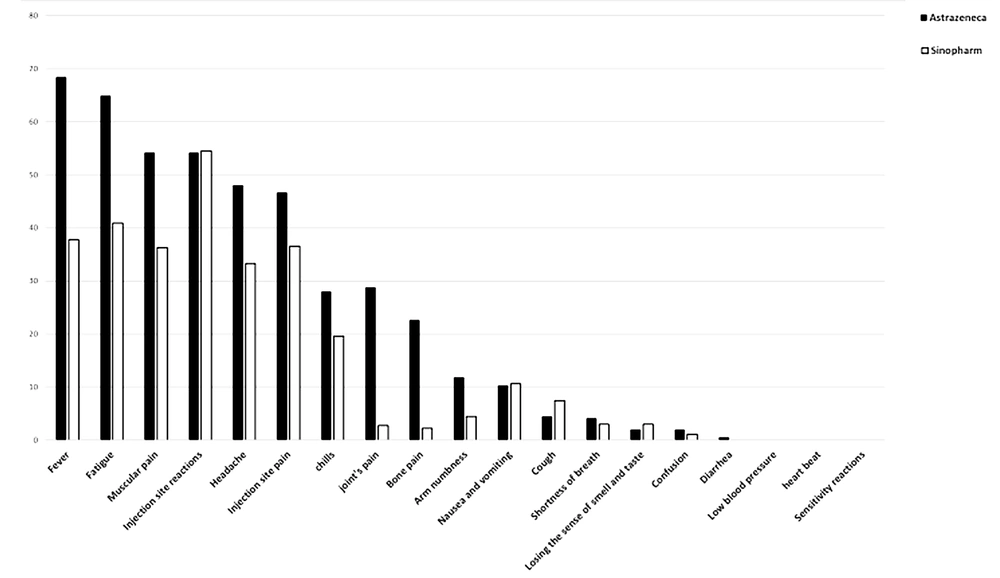
According to research conducted by the Centers for Disease Control and Prevention, common side effects after vaccination are pain, inflammation, and redness at the injection site, fatigue, fever, chills, muscle pain, headache, and nausea (38). According to experimental and other studies, the injections of each of the two doses of the above-mentioned three vaccines (ie, Sputnik V, AstraZeneca, and Sinopharm) have mild to moderate local and systemic side effects, including pain, redness, and inflammation at the injection site, fatigue, headache, chills, muscle pain, joint pain, and fever (39-41). According to a study performed by Kaur et al.,(15) most of the reported reactions after the injection of Sinopharm and Sputnik V vaccines were mild to moderate, and a small percentage was related to severe complications that all were resolved within 3 - 4 days.
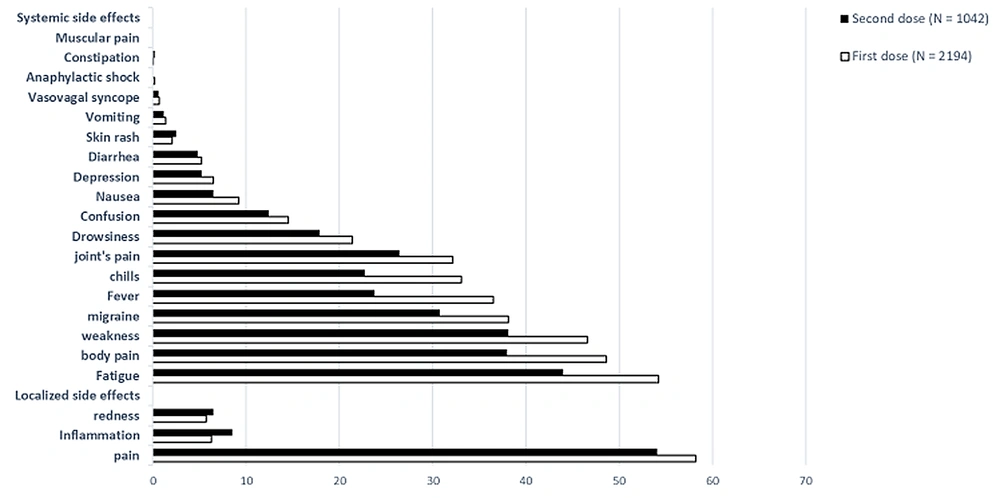
Common reported side effects of the Sputnik V vaccine, according to a study performed by Babamahmoodi et al.,(42) included injection site pain, fatigue, body aches, headache, fever, joint pain, chills, and drowsiness. Diarrhea, depression, skin rash, vomiting, and constipation are also less common symptoms. Rare side effects, such as increased heart rate, itching all over the body, shortness of breath, dryness and bad taste in the mouth, temporary hair loss, runny nose, and sore throat, were reported in less than 0.06% of study participants. These complications were significantly more frequently observed in women and younger individuals.
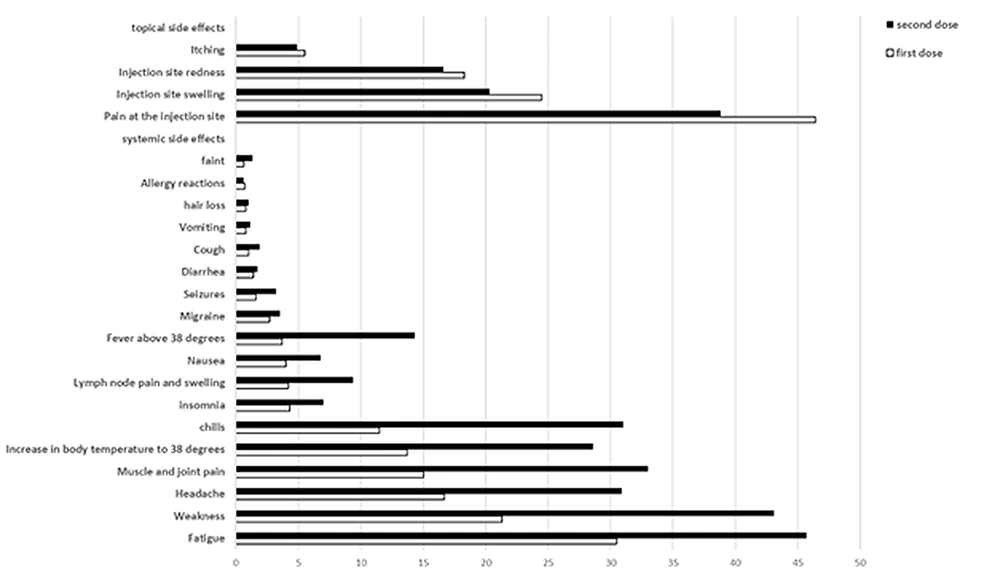
The onset of side effects in the subjects was more common within 12 - 24, 24 - 48, 12, and more than 48 hours to 7 days after vaccination, respectively. However, the duration of experiencing the side effects of the vaccine in most of the participants was less than 3 days (42). According to the findings of Montalti et al.’s study (43), the severity of local and systemic side effects after the second dose of the Sputnik V vaccine was higher than the first dose. Pagotto et al. (44) acknowledged that the side effects of the Sputnik V vaccine are more common in women and younger individuals, often occur 24 hours after injection and resolve in less than 3 days. According to another study, the side effects of the Sinopharm vaccine resolved within an average of 20 hours after injection (45). Moreover, the prevalence of Sinopharm side effects is in the form of pain at the injection site, fatigue, headache, and discomfort at the injection site, respectively. These side effects are more frequently pronounced in women (46). Other findings regarding the side effects of the Sinopharm vaccine are as follows:
(1) There was a clear linear relationship between vaccination complications and participants’ age. These complications are more common in individuals under the age of 49 years than in others (46).
(2) The side effects of the second dose of the vaccine were slightly higher than the first dose (47, 48).
(3) Despite the complication of fatigue, there was no significant difference in the possibility of discomfort and redness at the injection site, fever, and headache between men and women (46).
(4) No side effects were observed in 24% of the subjects after the first dose and 14% after the second dose (46).
(5) There was no difference between individuals with a history of COVID-19 and individuals with no history of the disease in the onset of symptoms after vaccination (45).
(6) No serious side effects or the need for hospitalization were observed after vaccination (26).
(7) All side effects after the injection of the Sinopharm vaccine were common, predictable, nonlife-threatening, and nonserious (46).
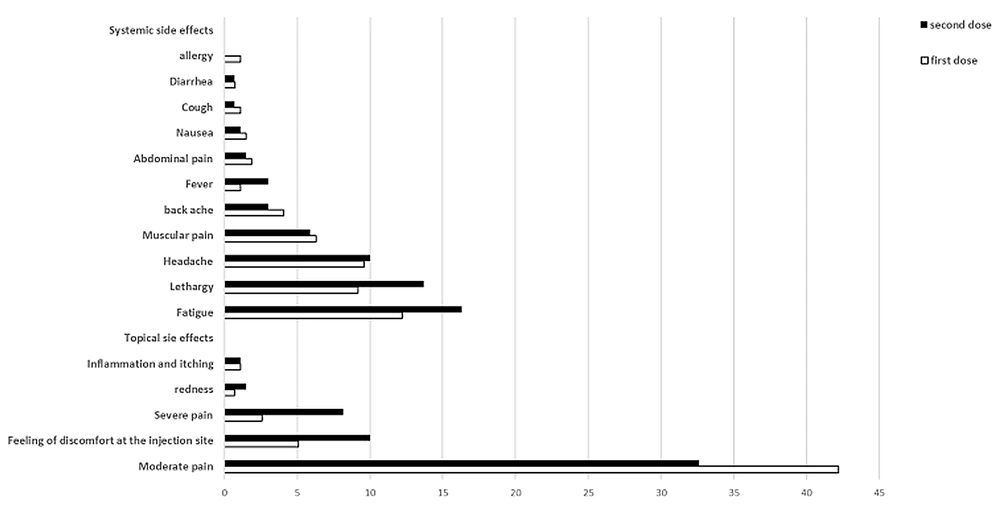
A study conducted by Menni et al. (4) showed that the adverse effects of the AstraZeneca vaccine are common and as follows:
Local symptoms: Feeling discomfort and pain at the injection site, warmth at the injection site, inflammation, redness, and itching
Systemic symptoms: Headache, fatigue, chills, joint pain, and fever (4).
In addition, uncommon symptoms, such as excessive sweating, swollen lymph nodes, pain, loss of appetite, and confusion, have been observed (49). According to the Indian Ministry of Health, the side effects of the AstraZeneca vaccine can last for up to 24 hours and, according to the Food and Drug Administration of India, from a few days to a week (50, 51). Other side effects of the AstraZeneca vaccine are as follows:
(1) Compared to men, the severity of complications was higher in women (52).
(2) The severity of complications in individuals under 50 years of age was reported to be higher than others (52).
(3) Individuals with a history of COVID-19 experienced relatively more complications after the vaccine (52).
(4) Individuals with chronic diseases and individuals undergoing medical treatment had slightly fewer local side effects and significantly fewer systemic side effects (52).
Thrombosis with thrombocytopenia syndrome is another rare complication of the AstraZeneca vaccine (53). Contrary to previous claims, no significant association was observed between the likelihood of this rare complication with age and gender, according to the latest update from the EMA. In this study, which was performed after injecting about 592 million doses of the AstraZeneca vaccine, 1503 cases (about 0.0002%) of this complication were reported, 43% of whom were reported in men and 37% in individuals over 60 years of age (54). According to the results of all studies, Figures 1-4 are separately presented to compare the prevalence of side effects of the first and second doses of AstraZeneca, Sputnik V, and Sinopharm vaccines.
4.1. Conclusions
This study investigated the short-term adverse effects associated with the most commonly used vaccines in Iran, namely AstraZeneca, Sputnik V, and Sinopharm. It was understood that pain at the injection site, fatigue, body pain, and weakness are the most common side effects after injecting the first and second doses of each of the aforementioned vaccines. It seems that the side effects observed following the injection of the AstraZeneca vaccine were more sensible than the other two vaccines. However, none of the reported side effects was life-threatening or worrying, and the benefits of vaccination are still very significant. Vaccination is the only preventive approach against COVID-19 and its consequences. The side effects of vaccination are signs that the vaccine is working well in the body.