1. Background
Toxicology research is employed to investigate the toxic effects, underline mechanisms, and medical care on hazardous materials, commonly chemicals. Much experimental work should be taken in the medical or life science laboratory. Before running the study, the researcher needs to be first educated about chemical safety, especially in handling a novel or highly toxic substance. Sulfur mustard (SM) is a synthetic vesicant agent that dates back to the early 19th century. It always imposes dangers to communities and individuals in public places and laboratories (1-4). Therefore, a less toxic SM analog, namely 2-chloroethyl ethyl sulfide (CEES), is employed to explore toxicological mechanisms and medical therapy for the category of vesicants (5-8).
CEES has a chemical structure similar to SM. Both are oily, hydrophobic, erosive, and quite different from many other skin-burning chemicals. Besides, they can be released into indoor air as a liquid spray (aerosol) or vapor. At room temperature, they are primarily a liquid hazard with low evaporation. With temperature increase, the vapor hazard increases. Although they are heavier than water, tiny droplets could still form a floating oil film on water surfaces while the droplets precipitate to the bottom and present a more prolonged hazard in contaminated areas (9). Although the vesicant characteristics have been well known, information about chemical safety in laboratory studies is still limited.
2. Objectives
This article summarizes the working procedure and experimental data to provide recommendations for other researchers to avoid occupational exposure to CEES in the laboratory study.
3. Methods
3.1. Sub-packaging and Sealing
CEES obtained commercially from Sigma (CAS number: 693-07-2, St. Louis, MO, USA) was about 5 or 25 mL in a brown glass bottle. Due to the small consumption in regular experiments, sub-packaging is necessary for easy and safe use. Noticeably, all procedures for handling CEES in the laboratory study should be taken in a qualified fume hood.
First, about 50 - 200 μL CEES was transferred into a 0.5 mL Polypropylene EP tube (Sigma, USA) and further stored in a refrigerator at 4ºC. The lid of the CEES containment (both glass bottle and EP tube) should be sealed by sealing film to avoid leakage. If not sealed, CEES could evaporate slowly even at 4ºC, leaking and polluting the laboratory. Since the CEES vapor in the EP tube may accumulate as tiny droplets on the top, to avoid splashing when opening the lid, brief centrifugation over 2,000 g is necessary to centrifuge the possible droplets. At all times, keep the tube up-right to make sure that the CEES liquid is always in the bottom of the EP tube.
3.2. Personal Protection
3.2.1. Eyes
The eyes are commonly the most vulnerable in laboratory study and are very sensitive to vesicants (10, 11). Thus, equipment is essential for eye protection. The researcher must wear safety glasses in the lab to protect eyes from possible injury by the vapor and splash of the vesicant or any other hazardous chemical.
3.2.2. Respiratory Tract
Although a fume hood could effectively reduce vapor dispersion, a qualified gas mask is still necessary to protect the respiratory tract. In our study, we selected 3M gas masks (3301CN, China) as personal respiratory protection equipment. The central part of this mask is a filter filled with irregular-shape activated carbon for adsorbing the organic vapor.
3.2.3. Hands
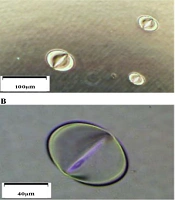
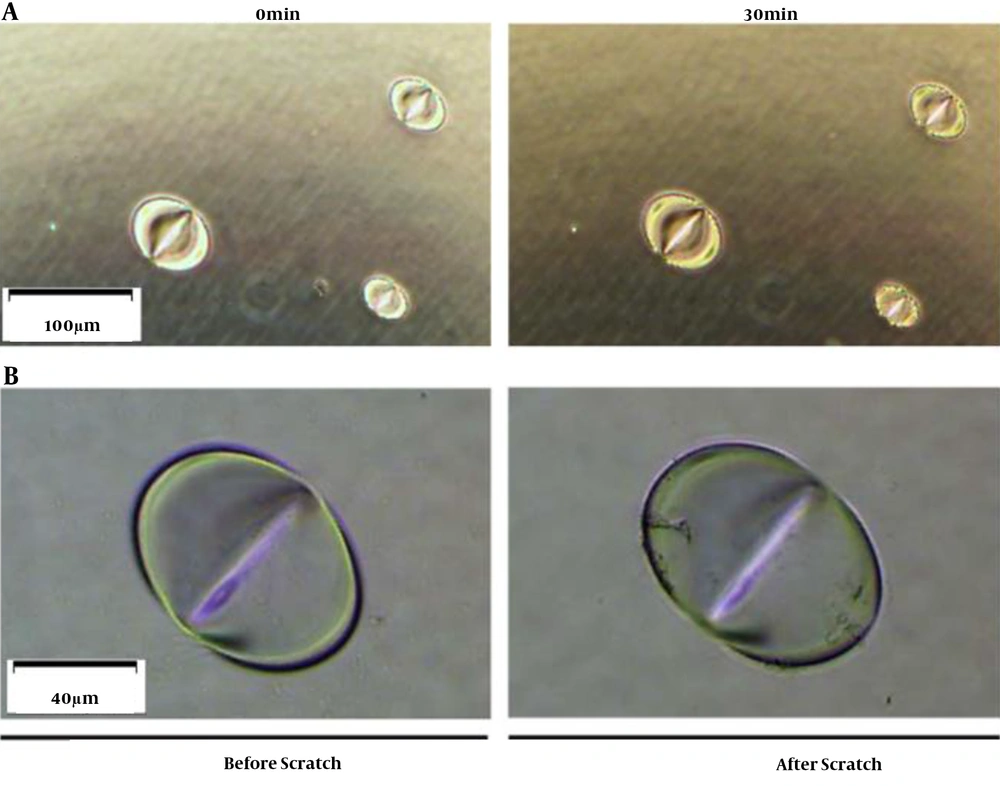
CEES is known to be erosive to the skin. Our previous study proved that pits are quickly formed once the plastic plate contacts a CEES droplet (Figure 1). The gloves are made with different materials, including polyethylene (PE), polyvinyl chloride (PVC), latex, and nitrile rubber. Because of different anti-chemical abilities, gloves need to be selected according to application. Nitrile is a polyisoprene extract from petroleum. Compared to PE, PVC, and latex, nitrile has a better anti-chemical performance to grease, xylene, aliphatic solvent, and some pesticide formulas. In handling CEES, the researcher is recommended to wear nitrile gloves to protect hands. Butyl rubber gloves also have great anti-chemical performances and can be used in the experiment, even in handling SM. However, due to being heavy and inflexible, the researcher is not suggested to wear them when handling CEES (Table 1). For most studies, wearing two pairs of nitrile gloves is enough to protect hands when handling CEES.
| Variables | PE | PVC | Nitrile Rubber | Butyl Rubber |
|---|---|---|---|---|
| Light | √ | √ | √ | - |
| Flexible | √ | √ | √ | - |
| CEES-resistant | - | - | √ | √ |
a √, yes or good; -, no or weak.
3.2.4. Others
Since contamination with CEES in a lab is usually through respiratory inhalation or hand contact, heavy and inflexible anti-chemical clothing is not usually employed during the study. General laboratory clothing is enough for body protection, and the clothing collar and cuffs should be tightened before the study to avoid naked skin exposure. After each experiment, washing clothing can clean many chemicals attached to the surface. However, please do not reuse the clothing polluted by CEES, but change and discard it after decontamination. At the end of the experiment, the researcher should use neutral detergents to wash hands carefully no matter if any possible hazards were contacted during the experiment.
3.3. Toxic Waste Management
Some agents, such as chlorinated lime, chloramine T, and dibasic tricalcium hypochlorite, could oxidate or chlorinate the vesicants to make them less harmful. Thus, toxic wastes like culture media, pipette tips, EP tubes, and gloves should be decontaminated before discarding them.
3.4. Decontamination
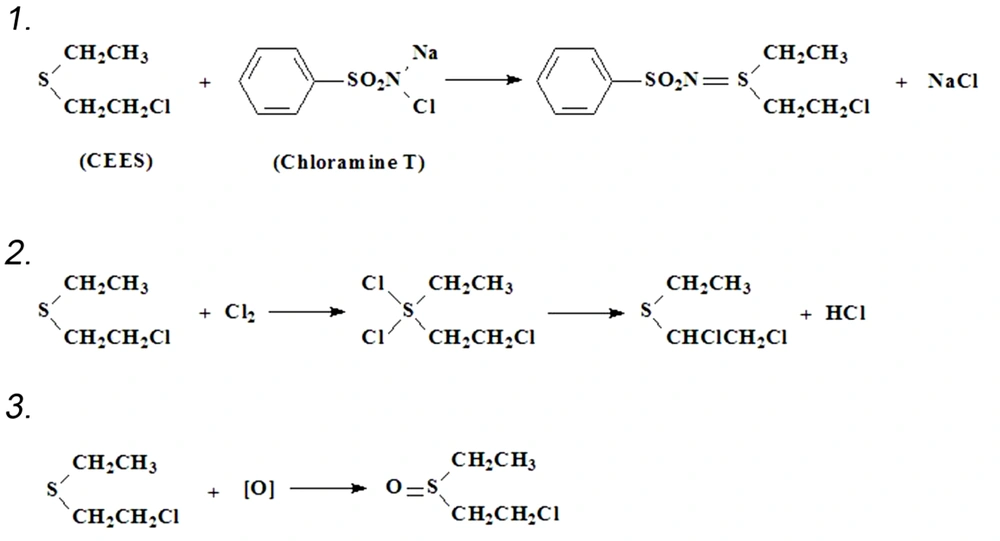
Since no feeling early after CEES exposure, the researcher should be vigilant and take action once in contact. The principle of decontamination is to prevent the contacted tissues and cells from vesicant damage by physical removal or (and) chemical reaction. Timely decontamination is vital in preventing or reducing vesicant damage since most of SM on the skin would be absorbed within 10 min (12), and decontamination for eyes has weak efficiency over 2 min after vesicant exposure. The visible droplets should be first wiped using cotton swabs or paper towels. After that, use a large amount of clean water to flush the area exposed to vesicant. Eyes need to be flushed with water for 5 to 10 minutes. Chloramine T, a chemical commonly employed in flushing root canals at a concentration of 2 %, is highly efficient in vesicant decontamination and recommended to be used before water flushing. The chemical reactions are shown in Figure 2.
3.5. Toxic Vapor Detection
A normal person can smell SM in the air at a concentration as low as 0.07 mg/m3. However, because the vesicant could reduce the sensitivity of human smelling (13), the researchers may not be sensitive enough for the vesicant vapor. Routine detection in a lab is forcefully required for surveilling the leaked vapor. We have been equipped with portable GC-MS (Mars-400 Plus, Focused Photonics, Hangzhou, China) in our lab for years. The instrument works by analyzing the pattern of chemical fragment peaks in pumped air to identify any vesicant in the air. The device’s detection sensitivity can reach the level of parts per billion (ppb, 1 μg/m3).
4. Discussion
Active sulfonium ions are formed once SM or CEES is dissolved in water (14, 15). They are unstable but highly toxic to cells as they can react with biomolecules like nucleotides and proteins. Both crosslinks (DNA-DNA or DNA-protein) and DNA/protein-adducts (CEES products) can be produced in SM-injured cells. Although less toxic than crosslinks, DNA/protein adducts are dominant in SM products (about 80%), and they may have long-term adverse effects on the poisoned cell (16, 17). Some known mechanisms are as follows: (1) CEES reduces RNA polymerase transcriptional activity to produce more RNA fragments of incomplete transcription (18); (2) only little CEES-formed O6-adducts in the alkylated DNA are removed by O-6-methylguanine-DNA methyltransferase (MGMT), which might cause cell mutation (19); (3) CEES inhibits cellular P450 enzyme system (20); and (4) CEES alkylates and inactivates many proteins/enzymes (21). In all, CEES is useful in the vesicant study for its less toxicity, but its chemical safety in laboratory study should be firstly and thoroughly educated to the new researcher.