1. Background
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the coronavirus acute respiratory syndrome 2 (SARS-CoV-2) (1). In late June, the first case of COVID-19 was reported in Wuhan, China, and now as of early June 2022, approximately 545 million worldwide and 7.3 million in Iran have been infected. Also, more than six million people have died in the world so far (2). Fever, dry cough, body aches, and shortness of breath are the most common symptoms reported in hospitalized patients. Anxiety and depression are also two common symptoms in hospitalized COVID-19 patients (3).
Although most cases have mild symptoms, some cases progress to pneumonia and multiple organ failure. Mortality rates are estimated at between 1% and 5% but vary with age and other health conditions (4). So far, no definitive and specific treatment has been identified for the disease, but some supportive therapies such as oxygen therapy, tummy tuck, diet therapy, and fluid therapy have shown some effective results (5).
Iranian medicine has been used for thousands of years to cure respiratory diseases such as pneumonia. Numerous studies have introduced medicinal plants and their possible mechanisms for reducing the signs and symptoms of COVID-19 (6-8). In traditional Iranian medicine, also known as Persian medicine, Hordeum vulgare or barley is used to treat many diseases. The barley-based remedy is useful for people with fever due to its cooling and cold properties. In a short time, it will lower the body temperature, and people who have frequent and long coughs are advised to drink barley-based remedies.
Survival analysis is a set of statistical methods for analyzing data in which the response variable is the time until the occurrence of an event. For example, the time from diagnosis to death or recurrence of the disease, the time until the first failure of the device in the factory, the time of divorce of a couple after marriage are examples of data that can be analyzed with survival analysis (9).
2. Objectives
This study aimed to evaluate the survival of adult patients hospitalized due to COVID-19 in the Mofatteh Hospital in Varamin, Iran, from 13 May to 10 July 2020.
3. Methods
The design and aims of this clinical trial were fully explained to all the eligible patients. The Medical Research Ethics Committee of Shahid Beheshti University of Medical Sciences approved the study protocol (code: IR.SBMU.RETECH.REC.1399.076).
Using block randomization method, a total of 70 patients were enrolled in the study. In addition to the usual hospital treatments for five days, the intervention group received barley-based medicine twice daily (200 ml in the morning and evening). The results of the study were evaluated daily for five days. If the patient was discharged earlier than five days, the outcome was assessed by the researcher. In this regard, a more complete explanation was published by Hasheminasab et al. (10).
The inclusion criteria were signing informed written consent, a positive nasopharyngeal reverse transcription polymerase chain reaction (RT-PCR) or lung computed tomography (CT) scan, and age over 18 years. We also excluded all pregnant and lactating women, as well as individuals with diabetes, high blood pressure, asthma, allergies, chronic kidney failure, chemotherapy, and immunodeficiency disease. Exclusion criteria were patient’s unwillingness to continue the study, ICU admission, use of herbal medicines or other traditional and complementary medicine, and tracheal intubation. Both intervention and control groups received routine care under the supervision of an infectious disease specialist.
One of the aims of survival data analysis is to compare survival times in two or more groups. For this purpose, a simple statistical test, log-rank test, is used. This test examines whether the survival of individuals in different groups is the same. The basic assumption of this test is the proportional hazards assumption, which can be rejected in different cases, especially when the survival curves studied at the logarithm level of the survival time intersect (11).
The data of the study was analyzed descriptively. Then, the median survival time was compared using Kaplan-Meier method, and the survival curve of patients was compared by log-rank test. A P-value less than 0.05 was considered as a significant level and SPSS software version 26 was used for analysis.
4. Results
Seventy eligible individuals were equally divided into two groups of control and intervention. Of the 70 patients, 7.1% (n = 5) patients died in the control group. Also, 4.2% (n = 3) in the intervention group and 2.8% (n = 2) in the control group were censored. In terms of gender, 43.8% (n = 14) were male in the intervention group and 51.5% (n = 17) were male in the control group. Details of the two variables of age and sex are shown in Table 1.
| Intervention | Control | |
|---|---|---|
| Gender | ||
| Male (%) | 43.8 | 51.5 |
| Female (%) | 56.2 | 48.5 |
| Age (mean ± SD) | 51.5 ± 16.3 | 61.9 ± 12.9 |
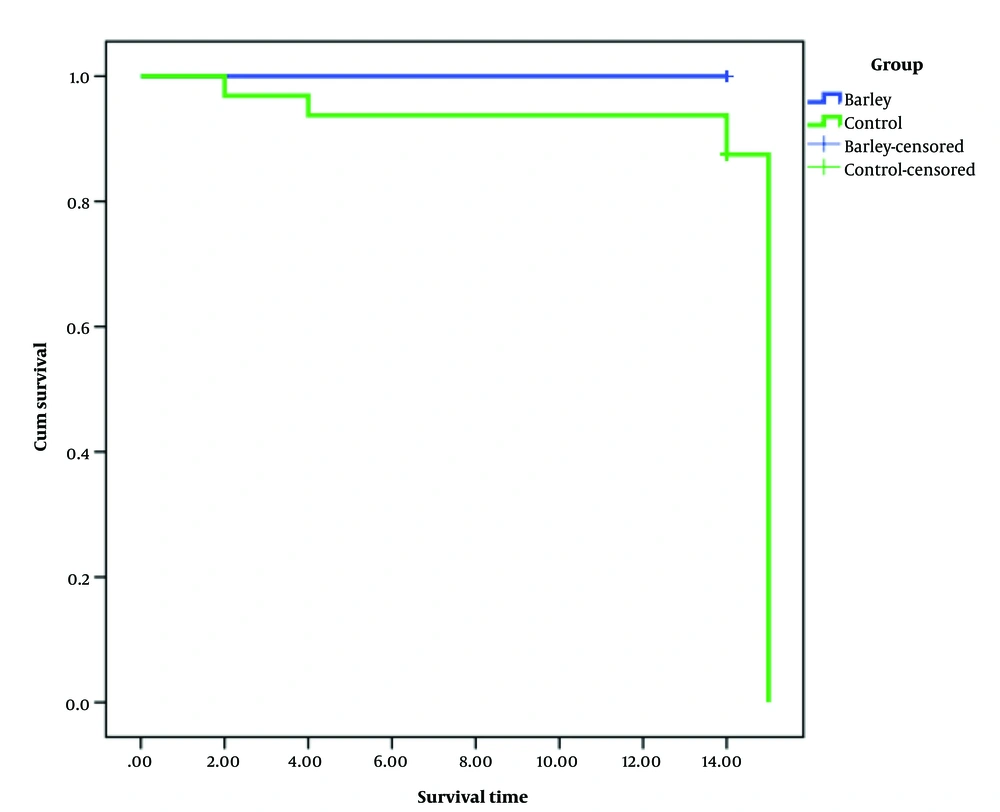
There was no significant difference between the groups in terms of gender (P = 0.124). Although individuals were randomly divided into intervention and control groups, there was a significant difference between the groups in terms of age (P = 0.006). Therefore, to eliminate the effect of age, subjects were divided into two groups (above 32 years and under 32 years). So, we used the classified Cox model for age. Therefore, in the Kaplan Meier method (SPSS software) in the Strata section, we placed the age variable and considered the appropriate cut-off point. Using the log-rank test, we observed that patients in the intervention group had more survival than patients in the control group (P = 0.04).
The mean survival time in the control and intervention groups was 13 and 14 days, respectively. According to Figure 1, there was no death cases in the intervention group, but in the control group, five patients died. However, further studies with larger sample sizes are needed to confirm the effectiveness of barley-based remedy in COVID-19 patients.
5. Discussion
The COVID-19 pandemic is not comparable to previous epidemics due to its rapid growth rate and high mortality rate. Researchers around the world are working to treat COVID-19, and they are using all modern medical therapies and traditional and complementary medicine to prevent and treat COVID-19 patients. An individual’s nutritional status can modify infectious diseases and related inflammatory processes by altering the immune system (12).
Barley juice extract called beer has been used for a long time in the treatment of fever and cough. Goupy et al. reported the effectiveness of barley juice extract on influenza virus (13). Also, Derakhshan et al. showed that barley juice extract can be an effective treatment to reduce the symptoms of allergic rhinitis (14).
A randomized clinical trial is the most definitive tool of clinical research to evaluate the effectiveness of new therapies in human subjects. Statistical methods for the analysis of survival data play a pivotal role in the analysis of clinical trial data (15). If the purpose is to describe survival time without considering auxiliary variables, nonparametric methods such as Kaplan Meier estimator or life table are used. In Kaplan Meier's estimator, the occurrence of incident events is considered insignificant, and the occurrence of each event in a short period of time is considered. Therefore, this method is useful when the number of data is small and measured with high accuracy.
So far, no research has been done on the survival of COVID-19 patients with barley-based drug intervention. However, several studies have been conducted on the survival of COVID-19 patients with nutritional interventions. Annweiler et al., in a quasi-experimental study, evaluated COVID-19 patients in a nursing home in southeastern France in March 2020. In this study, there was no significant difference between the two groups in terms of age and sex, and the time elapsed until death was calculated according to the Kaplan-Meier method and compared with log-rank test. The results showed a significant difference between the two groups. As a result, it was found that vitamin D3 supplementation taken during or just before COVID-19 was associated with the severity of COVID-19 and increased survival (16). The results of this study are consistent with our results in which nutritional interventions increased the survival of COVID-19 patients.
Doaei et al. conducted a double-blind, randomized clinical trial in Rasht, Iran, on COVID-19 patients from May to July 2020. They reported that omega-3 supplementation improved different levels of respiratory and renal function parameters in COVID-19 critically ill patients (17). The results of this study are also consistent with our results because in both studies, nutritional intervention affected the mortality of COVID-19 and increased the survival of COVID-19 patients.
The interaction between nutrition and the immune system is well known. Therefore, any nutritional imbalance affects the competence and integrity of the immune system. In our study, there was no significant difference between the groups in terms of sex and age.
In examining survival data, the Kaplan-Meier non-parametric method is used if the purpose is to describe the survival time without considering auxiliary variables. In this study, using the Kaplan-Meier method and log rank test, there was a significant difference between the intervention and control groups.
5.1. Conclusions
We found that barley-based remedy could increase the survival time of COVID-19 patients. So far, no research has been conducted on the survival of COVID-19 patients with barley-based remedy intervention. In this study, the survival rate of COVID-19 patients who received formulation based on Hordeum vulgare was higher than patients who did not receive it.