1. Background
The prevalence of obesity and being overweight are of the biggest public health challenges of the present century and bring about clinical and public health burdens worldwide. Thus, it is acknowledged that national programs for the prevention and treatment of overweight, obesity, and its related comorbidities should be prioritized (1). It is also argued that being one of the most important factors affecting energy metabolism, physical activity has an important role in reducing the risk of obesity and controlling body weight by altering the balance between energy intake and energy expenditure (2, 3). Regular physical activities are also beneficial to ones health as they prevent various diseases such as cancer, cardiovascular diseases, diabetes, obesity, and cognitive diseases (4-6).
The neurotrophins, the most important trophic factors in the nerve system, consist of nerve growth factor, brain-derived neurotrophic factor, neurotrophin-3, and NT-4/5 (7). Brain-derived neurotrophic factor, first discovered and purified in 1982 from the brain of a pig, plays a major role in neurogenesis and neuronal-death protection, and also positively influences brain plasticity by improving learning and memory (8). Its secreted form has the molecular weight of 27.8 kDa and consists of 247 amino acids (9).
Brain-derived neurotrophic factor in the neural system exerts its biological effects via 2 types of receptors including the pan-neurotropin receptor p75 and the tyrosine kinase receptor B receptor (10). Brain-derived neurotrophic factor expression has been reported in various parts of the human brain including the hippocampus, amygdala, cerebral cortex, cerebellum, septum, and the nucleus of the solitary tract. It also is found in other body tissues such as the heart, muscles, kidney, lungs, and testes (11).
Aside from its effects on synaptic plasticity in the central nerve system (CNS), it involves elements of cellular energy metabolism and in the periphery it takes part in metabolic processes such as enhancing lipid oxidation in the skeletal muscle via activating adenosine monophosphate-activated protein kinase (12, 13). Therefore, it plays a critical role in central and peripheral energy metabolism as studies reveal that brain-derived neurotrophic factor levels are lower in patients with neurodegenerative diseases including Alzheimer’s disease and major depression. In addition, obese people and patients with type 2 diabetes have lower levels of brain-derived neurotrophic factor (14, 15).
Physical activity is one of the important factors affecting energy metabolism, and brain-derived neurotrophic factor seems to be regulated by exercise and physical activity. However, variations in the types, intensity, and duration of physical activity yield in different physiological responses.
Ruh et al., having investigated the effects of obesity and aerobic exercise training on brain-derived neurotrophic factor, reported that at baseline, the obese group had significantly lower brain-derived neurotrophic factor levels than the normal-weight group; nevertheless, after the exercise training, the increase of brain-derived neurotrophic factor in the obese group was significantly higher than in the normal-weight group. They also concluded that obesity reduces the Brain-derived neurotrophic factor level but exercise training increases its levels (16). Nevertheless, Babaei et al., reported that brain-derived neurotrophic factor levels in athletes are lower than physically inactive people (17).
Regular physical activity is beneficial to health and prevents many diseases; however, using aerobic or resistance exercise programs alone is not sufficient to take advantage of physical activity. Thus, American College of Sports Medicine recommends both of these activities. Therefore, this study investigated the acute and chronic effects of combined training on brain-derived neurotrophic factor levels and the association between brain-derived neurotrophic factor levels and anthropometric variables in overweight young men.
2. Methods
2.1. Subjects
Volunteers’ levels of physical activity and disease records were determined through the special questionnaire. A total of 20 qualified volunteers (overweight, aging 20 - 25 years, and with body mass index 25 - 30) were selected and randomly divided into equal (n = 10) control and experimental groups. It is worth mentioning that all stages of the study accorded the Declaration of Helsinki and were approved with the research ethics committee of sport Sciences research institute with the code of IR.SSRI.REC.1395.109.
2.2. Anthropometric Measurements
One week before starting the training in the gymnasium, the participants were briefed on the objectives and methodology of the study and were familiarized with the movements and devices. Their weights, heights, body fat percentages, one repetition maximum (1 RM) of various movements, and maximum oxygen consumptions were also recorded. Specifically, the subjects’ heights were measured while standing without shoes against the wall. Their weights were also measured using a digital scale while they had minimal clothing. Their body mass indices were also calculated by dividing weight (kg) by square of the height (m2). Their skin fold thicknesses were also measured by caliper according to the Jackson and Pollock method at 3 sites (chest, abdominal, and thigh) in the right side of body to estimate body fat percentage. It deserves mentioning that all measurements were done 3 times in each area by 1 person and the average of 3 measurements was considered as the final record. For the sake of reliability and validity, all measurements were done at the same time, preferably morning (after the overnight fasting).
2.3. Training Protocol
This study involved a combined training program (strength and endurance) 3 times a week for 8 weeks. The participants performed 10 minutes of warm up exercises including jogging and stretching movements, and ran for 20 minutes by 50% to 85% of maximum heart rate (training started with 50% of intensity and it was increased by 5% each week) at the beginning of each session. Then, they performed strength training for large muscles of the upper and lower body, which included lat pull-down, bench press, leg press, barbell shoulder, and knee flexion with 50% to 80% of one repetition maximum (training started with 50% of intensity and it was increased by 5% each week). They performed 3 sets with 10 repetitions (with 1-minute rest interval between sets and 2-minutes rest interval between exercises). Training sessions ended with a 10-minute cooling-down though walking slowly (18).
2.4. Blood Sampling
In the current study, blood samplings were done at 3 different times including pre-training, after the acute training, and 48 hours after the end of the training period (chronic). Blood samples were taken from the antecubital veins of the overnight fasting subjects by a laboratory expert after their sitting on a chair for 15 minutes. The blood samples were then kept at room temperature for 1 hour to be clotted. Then, they were centrifuged and serums were kept at -80°C until final measurements. Brain-derived neurotrophic factor concentrations were measured through the commercially available ELISA kit specific for human samples based on the instruction of manufacturer (EASTBIOPHARM, Hangzhou Co. Ltd, China).
2.5. Statistical Analysis
The data were analyzed through the Statistical Package for Social Sciences (SPSS version 16) and the level of significance was determined at P ≤ 0.05. As the results of the Kolmogorov-Smirnov tests proved normality of the data, ANOVA 2 × 3 with repeated measures and the Bonferroni post hoc test were run for analyzing the data. Independent t-test was also used for testing the differences between the 2 groups in different measurement times. Pearson correlation tests also probbed the relationship between Brain-derived neurotrophic factor and anthropometric variables.
3. Results
Table 1 presents means and standard deviations of physical and anthropometric characteristics (including age, height, weight, body mass index, and body fat percentage) of 2 groups.
| Variable | Group | ||
|---|---|---|---|
| Measuring | Control | Experimental | |
| Age, y | Pre-training | 22.3 ± 1.5 | 22.7 ± 1.5 |
| Height, cm | Pre-training | 178.8 ± 3.1 | 180 ± 4 |
| Weight, kg | Pre-training | 86.6 ± 2.6 | 87.3 ± 3.9 |
| Post-training (chronic) | 86.7 ± 2.3 | 86.4 ± 3.4 | |
| BMI, kg/m2 | Pre-training | 27.1 ± 0.8 | 26.9 ± 0.7 |
| Post-training (chronic) | 27.1 ± 0.9 | 26.9 ± 0.6 | |
| Body fat, % | Pre-training | 22.8 ± 1.6 | 22.6 ± 1.3 |
| Post-training (chronic) | 22.7 ± 1.5 | 21.5 ± 1.5 | |
Means and Standard Deviations of Physical and Anthropometric Characteristics of the Subjects (N = 10)a
Differences in anthropometric characteristics of the subjects at different times of measurement, i.e., before and after the training period, were probed through paired t-tests, which indicated that only the fat percent in the experimental group was reduced significantly and there were no significant differences in other characteristics of the participants.
The average food intakes of the subjects were recorded 3 days before the blood samplings and analyzed with food analyzer software (NUTRITION 4) (Table 2). It should be mentioned that a copy of the subjects’ food intake forms completed in pre training were given to them before the final blood sampling so as to allow their following the forms.
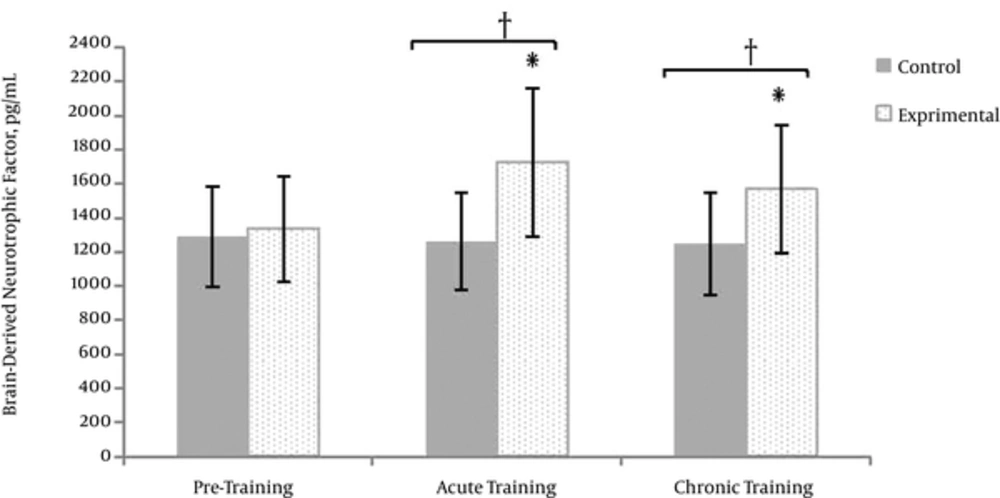
Significant differences were found between brain-derived neurotrophic factor levels at different times of measurements (F = 10.68, sig = 0.001). There was also a significant interaction between groups and times of measurements (F = 14.53, sig = 0.001).
| Variable | Group | ||
|---|---|---|---|
| Stage | Control | Experimental | |
| Energy, kca | Primary | 3187 ± 143 | 3241 ± 179 |
| Secondary | 3169 ± 252 | 3272 ± 204 | |
| Carbohydrate, gr | Primary | 451.1 ± 58 | 460 ± 21.7 |
| Secondary | 442.8 ± 37 | 455 ± 33.1 | |
| Protein, gr | Primary | 129 ± 48 | 131.7 ± 29 |
| Secondary | 132 ± 39 | 139.8 ± 36 | |
| Fat, gr | Primary | 121.1 ± 19 | 127.3 ± 48 |
| Secondary | 129.7 ± 31 | 120.3 ± 37 | |
Means and Standard Deviations of Food Intake Analysis (N = 10)a
Within group changes for each of the groups were tested through separate ANOVA repeated measures. The control group had no significant difference between the variances of brain-derived neurotrophic factor levels at different times of measurements. However, the brain-derived neurotrophic factor levels of the experimental group were significantly different at different measurement times. The differences were further probed running Bonferroni post hoc test the results of which revealed a significant increase in Brain-derived neurotrophic factor levels after the acute and chronic training, as compared to the pre-training. (P ≤ 0.05) (Figure 1).
The differences between the Brain-derived neurotrophic factor levels of the experimental and control groups at different times of measurements were tested through the independent t-test (Table 3). The results indicated a lack of differences in the concentrations of Brain-derived neurotrophic factor levels of the 2 groups at the pre-training; however, they had different levels of brain-derived neurotrophic factor levels after the acute and chronic training as the experimental group had the higher concentration of brain-derived neurotrophic factor than the control group (P ≤ 0.05).
The Comparison Between the Brain-Derived Neurotrophic Factor of the experimental and Control Groups at Different Times of Measurements
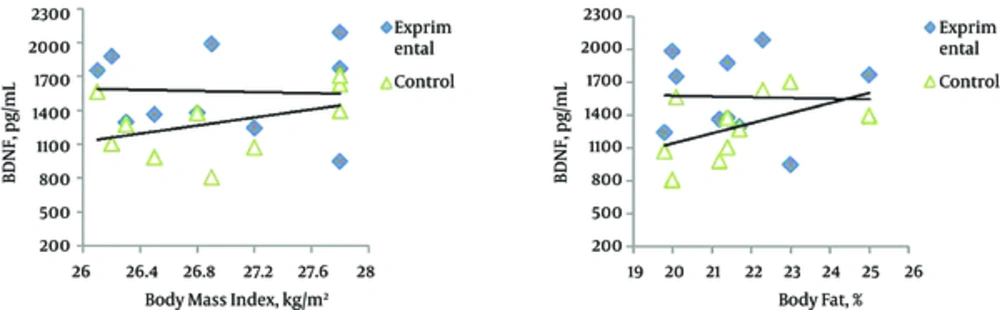
At the end of training protocol, the relationship between brain-derived neurotrophic factor and anthropometric characteristics including body mass index and fat percentage of the subjects were assessed using Pearson’s correlation tests, the results of which revealed no significant correlation between the anthropometric variables and brain-derived neurotrophic factor levels (Figure 2).
4. Discussion
This study was carried out to investigate both the acute and chronic effects of combined exercise training on brain-derived neurotrophic factor levels and the relationship between brain-derived neurotrophic factor levels and anthropometric variables in overweight young men. Our findings indicated that the acute and chronic combined exercise training (strength and endurance) significantly increased brain-derived neurotrophic factor levels. However, the concentrations of brain-derived neurotrophic factor levels were not related to anthropometric variables including the body fat percentage and body mass index.
Variations in the levels of brain-derived neurotrophic factor due to the various types of physical activities have been investigated and contradictory results have been reported. Some studies reported increased brain-derived neurotropin levels as a result of exercise, which is in line with the findings of this study (19-22). Brain-derived neurotrophic factor can cross the blood-brain barrier in both directions, i.e. from the brain to the periphery and from the periphery to the brain. Furthermore, brain-derived neurotrophic factor levels in the brain is associated with the serum concentration. Thus the serum level of brain-derived neurotrophic factors may reflect its brain level (17). During physical activity and resting condition, the brain contributes to approximately 70% - 80% of the circulating brain-derived neurotrophic factor levels, and thus acts as its main source. However, other peripheral sources such as platelets, vascular endothelial cells, and skeletal muscles also contribute to the brain-derived neurotrophic factor levels (23).
The increase in the brain-derived neurotrophic factor levels after the acute physical activity can be due to its enhanced release from different tissues including the active muscles and brain. Also, the increase in its levels after the chronic training may be caused by the increase in gene expression and activating the transcription pathways. Therefore, increase in the Brain-derived neurotrophic factor levels, aside from its roles in neurogenesis and brain health, plays a critical role in central and peripheral energy metabolism through hypothalamus pathways.
Inconsistent with our findings, some studies reported a decrease or no changes in the levels of brain-derived neurotrophic factor after physical activity (24-27). This can be due to the effect of type and intensity of exercise, age and physical fitness levels and health status of individuals on the synthesis, as well as release of brain-derived neurotrophic factor into blood stream. In the same vein, Uysal et al. reported that brain-derived neurotrophic factor levels were higher in the voluntary exercise than in the involuntary exercise (19, 22). Cho et al. reported that the concentration of brain-derived neurotrophic factor level increased significantly after long term combined exercise in mid-aged women (19). In addition, Schmolesky et al. examined the effects of the intensity and duration of aerobic exercise on brain-derived neurotrophic factor levels and stated that vigorous intensity and long duration exercises caused the greatest elevation in brain-derived neurotrophic factor levels (28).
Also, lack of change in the amount of brain-derived neurotropic factor can also be due to the greater tissue absorption and effective clearance by tissues or transmission into the brain.
Also, some authors reported that reduction in serum brain-derived neurotropic factor reflects a kind of adaptation to the physical activity, down regulation of brain-derived neurotropic factor synthesis, reduction in its releasing mechanism, or more consumption by central nerve system (17, 29). In addition, after regular physical activity, plasma volume increases by 10 to 20%, and this may explain the lower levels of brain-derived neurotrophic factor in athletes (17).
Babaei et al. documented that acute aerobic and anaerobic exercises elevated brain-derived neurotrophic factor levels in athlete and sedentary groups as compared to the resting state. Also, the athlete group had lower basal brain-derived neurotrophic factor levels than the control group. They suggested that long-term habitual exercise is associated with lower peripheral brain-derived neurotrophic factor levels (17).
As for the relationship between the brain-derived neurotrophic factor levels with anthropometric variables, we found no significant relationships between them. Furthermore, Swift et al., in line with our results, reported that brain-derived neurotrophic factor was not associated with fitness, body composition, and anthropometry in individuals with type 2 diabetes (27). However, Babaei et al. found a positive correlation between brain-derived neurotrophic factor level and body mass index and attributed it to the compensatory metabotropic, rather than a neurothrophic, role of peripheral brain-derived neurotrophic factor (17).
The increase of brain-derived neurotrophic factor levels following an acute exercise seems to be caused by its elevated release from different tissues into the blood stream on the one hand and its greater tissue absorption on the other hand. In most studies, brain-derived neurotrophic factor levels increased significantly due to acute exercise; however, its concentrations returned to baseline at post-exercise, showing a fast disappearance rate of circulating brain-derived neurotrophic factor levels after the cessation of exercise. Similarly, it is reported that brain-derived neurotrophic factor levels decreased below baseline concentration within 3-hours after acute cycling exercise.
4.1. Conclusion
Both acute and chronic combined training caused an increase in brain-derived neurotrophic factor levels. Acute exercise may increase the brain-derived neurotrophic factor levels possibly due to the release and secretion of various tissues such as brain or skeletal muscles into blood stream while chronic exercise may enhance its levels by increasing gene expression and activating transcription pathways. Apart from playing important roles in neurogenesis, cognitive functions, and preventing neurodegenerative diseases, increasing levels of brain-derived neurotrophic factors may plays a metabotropic role through the hypothalamic pathway and controls body weight and energy homeostasis in overweight men.