1. Background
Gastroesophageal reflux disease (GERD) is one of the most common diseases of the digestive system, which is caused by the dysfunction of the lower esophageal sphincter and also by causing a disturbance in the clearing of stomach acid (1, 2). This disease is especially common in developed countries. Its prevalence is 18 - 27% in North America, 8 - 25% in Europe, 23% in South America, 11% in Australia, and 7 - 2% in East Asia (3).
Compared to other Asian countries, it seems that GERD is more common in Iran, and its prevalence is similar to Western countries. The prevalence of 21.2% in a study in Tehran is the best estimate for reflux in Iran (1). The estimate of 21.5% in one study, and 25.5% in one study in Shahrekord, can indicate the high prevalence of the disease in Iran. The annual prevalence rate in one of the studies is estimated to be more than 85% (4). This high prevalence affects not only the quality of life but also the economy. The financial burden of GERD in the United States of America may directly reach 9 - 10 billion dollars annually. This amount is apart from the indirect costs that are imposed on society due to the reduction of productivity in daily work (3).
Gastroesophageal reflux disease is a disorder in which the reflex passage of stomach contents into the esophagus leads to signs such as stomachache, chest pain, hoarseness, asthma, painful throat, globular feeling, and tooth decay (5). The frequent presence of gastric contents with pH < 1 in the mouth may create a persistent acidic situation that can harm the mouth's solid and soft tissues. A sufficient amount and quality of salivary secretions are necessary to protect the teeth and mucous membranes of the mouth, pharynx, and esophagus (5).
In the pathophysiology of GERD, dysfunction of various mechanisms known as the "anti-reflux barrier" occurs, one of the most obvious of which is the dysfunction of the autonomic nervous system, especially the parasympathetic nerves, so that parasympathetic activity is impaired in all patients with GERD (6, 7). Inflammation motivates releasing of acetylcholine (Ach) from the parasympathetic end. Ach can suppress inflammation in both the central nervous system (CNS) and peripheral tissues. Cholinesterases include a group of enzymes that hydrolyze acetylcholine and other choline esters. Cholinesterase activity is reduced in various chronic and severe inflammatory diseases (8-12).
Today, although the ambulatory pH monitoring method is the diagnostic gold standard, its sensitivity for esophageal reflux is lower than for non-esophageal reflux. Studies have shown that there are day-to-day changes in pH meter results, with one patient having a regular test on one day and an abnormal test on another day. Finally, none of the above diagnostic methods have been proven as a reliable tool for GERD, and the search for more accurate diagnostic methods is necessary (13, 14).
2. Methods
In a cross-sectional study, 30 patients with GERD referred to the gastroenterology clinic of Imam Reza Hospital who had a return of stomach contents to the mouth and pharynx and grade B or higher esophagitis based on the Los Angeles classification and 30 healthy individuals selected from the people who visited the hospital for annual monitoring and were healthy were included in the study. Two milliliters of venous blood were taken from all the participants and poured into the gel clot tube. Clotted blood was centrifuged at 5,000 rpm for ten minutes. The samples were stored at -70°C. The cholinesterase activity kit was purchased from Pars Azmoun (Karaj, Iran). Photometric measurement was done according to the manufacturer’s instructions. Samples were assayed at least twice. The data were analyzed in SPSS software version 25 with the unpaired student t-test and receiver operating characteristic (ROC) curve analysis.
3. Results
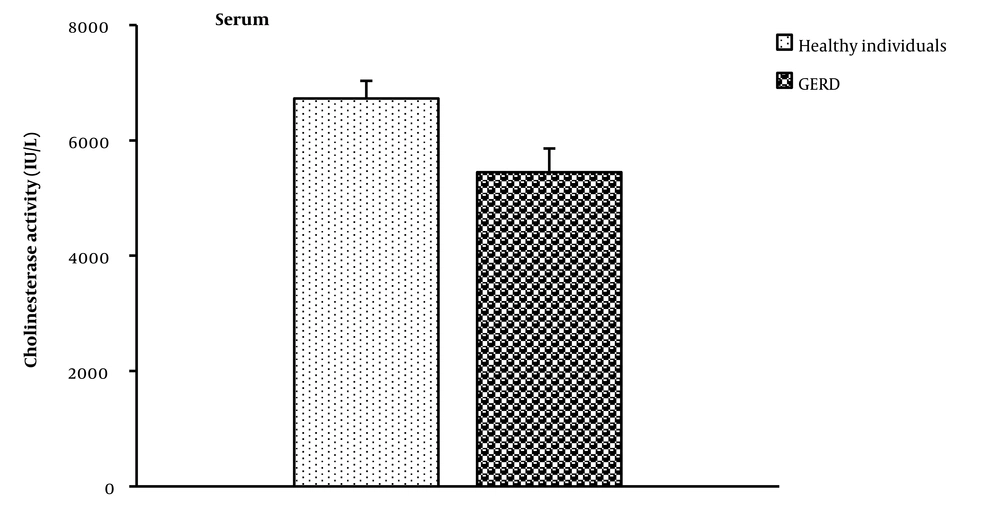
The average serum cholinesterase activity in the GERD group was lesser than that of healthy subjects (P = 0.001) (Figure 1).
The results of ROC curve analysis showed that the evaluation of cholinesterase activity in serum could be used to distinguish patients with GERD from healthy individuals. The serum cholinesterase activity cut-off point for distinguishing patients with GERD from healthy people was 5,637 IU/L with 61% sensitivity and 54% specificity (P = 0.027).
4. Discussion
Gastroesophageal reflux disease is one of the most public digestive diseases, and its prevalence is relatively high in Iran. Its economic burden is high, so it is estimated that Americans spend more than 10 billion dollars per year on proton pump inhibitors (PPIs) for its treatment (15). The reduction of the quality of life and the occurrence of many problems such as absenteeism, reduction of leisure time programs, and increase of problems related to doing household chores are among the problems of patients with GERD. However, most cases of GERD (79.2%) are non-complicated (16).
Today, there are various methods for diagnosing this disease, some of which do not have sufficient sensitivity and specificity. Therefore there is still no gold standard diagnostic test for GERD, and the diagnosis is based on a combination of PPI tests, ambulatory pH monitoring, and esophagogastroduodenoscopy (EGD). Other methods, such as barium esophagogram, high-resolution esophageal manometry, and gastric emptying studies can help rule out other diseases that may contribute to or mimic the signs of GERD (17). Most of these diagnostic methods are expensive. Therefore, finding a solution to diagnose this cost-effective disease seems necessary. In the pathophysiology of GERD, dysfunction of various mechanisms known as the "anti-reflux barrier" occurs, one of the most obvious of which is the dysfunction of the autonomic nervous system, especially the parasympathetic nerves, so that parasympathetic activity is impaired in all patients (6, 7). Inflammation motivates the “cholinergic anti-inflammatory pathway,” which motivates the release of Ach (18). Ach can suppress inflammation in both CNS and peripheral tissues (19). Acetylcholine affects macrophages through the α7 nicotinic receptor, which results in stopping the NF-κB nuclear transmission signaling pathway, which leads to a decrease in the making of pro-inflammatory cytokines. In this way, the cholinergic system with a reflex mechanism can quickly recover inflammation by getting evidence from various body parts (11, 18).
Cholinesterase activity is reduced in various chronic and severe inflammatory diseases (9-13). Since cholinesterases are made in the liver, their amount decreases in liver patients and malnutrition (19-21). Also, cholinesterase activity in acquired immunodeficiency syndrome (AIDS) (22), burns (20), in patients with gingivitis and periodontitis (23), cancer (24), brain damage (25), ischemic stroke (26), anorexia nervosa (27), Alzheimer’s disease (28) and multiple sclerosis (MS) (29) is reduced. Regulating the systemic level of Ach requires continuous control of the balance between the production of Ach by the vagus nerve and its hydrolysis by acetylcholinesterase and butyrylcholinesterase. Since the serum levels of cholinesterase reflect fluctuations in the body’s total capacity to hydrolyze Ach; therefore, the reduction in the serum level of cholinesterase is related to the downregulation of cholinesterase activity as a compensatory reaction of the body to increase the anti-inflammatory activity of acetylcholine (18).
The acid and pepsin contents of the stomach can cause damage to the esophagus and mouth and then lead to an increase in inflammatory factors and the occurrence of inflammation. Since the level of cholinesterase activity decreases in inflammatory diseases, it can justify the decrease in the serum level of cholinesterase activity in patients with GERD. The results showed that the cut-off point of cholinesterase activity in serum was significant for distinguishing GERD. Therefore, assessing cholinesterase activity may be helpful in the diagnosis of GERD.
4.1. Conclusions
It seems that the amount of cholinesterase activity is reduced in the serum of patients with GERD.