1. Background
Neutropenia is a common unfavorable side effect in chemotherapy patients, which results in increased mortality and morbidity. The incidence rate of hospitalization in chemotherapy-induced neutropenic patients was 0.0078%, and the mortality rate was 6.8%. It also imposes a heavy economic burden on the health system due to prolonged hospitalization and expensive drugs (1, 2). Thus, prevention of infection in these patients could be rational. Many studies showed a great advantage of antibacterial prophylaxis in high-risk neutropenic patients, especially using a fluoroquinolone drug (FQ) (3-5). These drugs' old and new generations are relatively available and inexpensive, with relatively low side effects. FQ drugs appear to be a good choice for prophylaxis. A study on 172 patients with acute myeloblastic leukemia (AML) showed that ciprofloxacin was more effective than colistin in reducing FN (6). Another study showed that levofloxacin prophylaxis decreased bloodstream infection in hematopoietic stem cell transplantation (HSCT) patients. However, the risk of BSI did not differ in patients with lymphoma (7). One study found similar results in prophylaxis with levofloxacin in patients with acute leukemia (8). Initiating ciprofloxacin concurrently with chemotherapy in acute leukemia resulted in delayed empirical treatment and overall antibiotic usage (9). A similar result was observed in reducing febrile neutropenia (FN), bacteremia, and hospitalization in neutropenic patients who received ciprofloxacin (10). Although these studies agreed on the use of prophylaxis with FQ drugs in neutropenic patients, one study failed to show a significant reduction in infection-related mortality (11).
2. Objectives
Regarding increasing antimicrobial resistance to fluoroquinolones in recent years, different results of the previous studies, and other possible differences in our population, we aimed to evaluate the efficacy of oral ciprofloxacin in preventing FN among patients with chemotherapy-induced neutropenia.
3. Methods
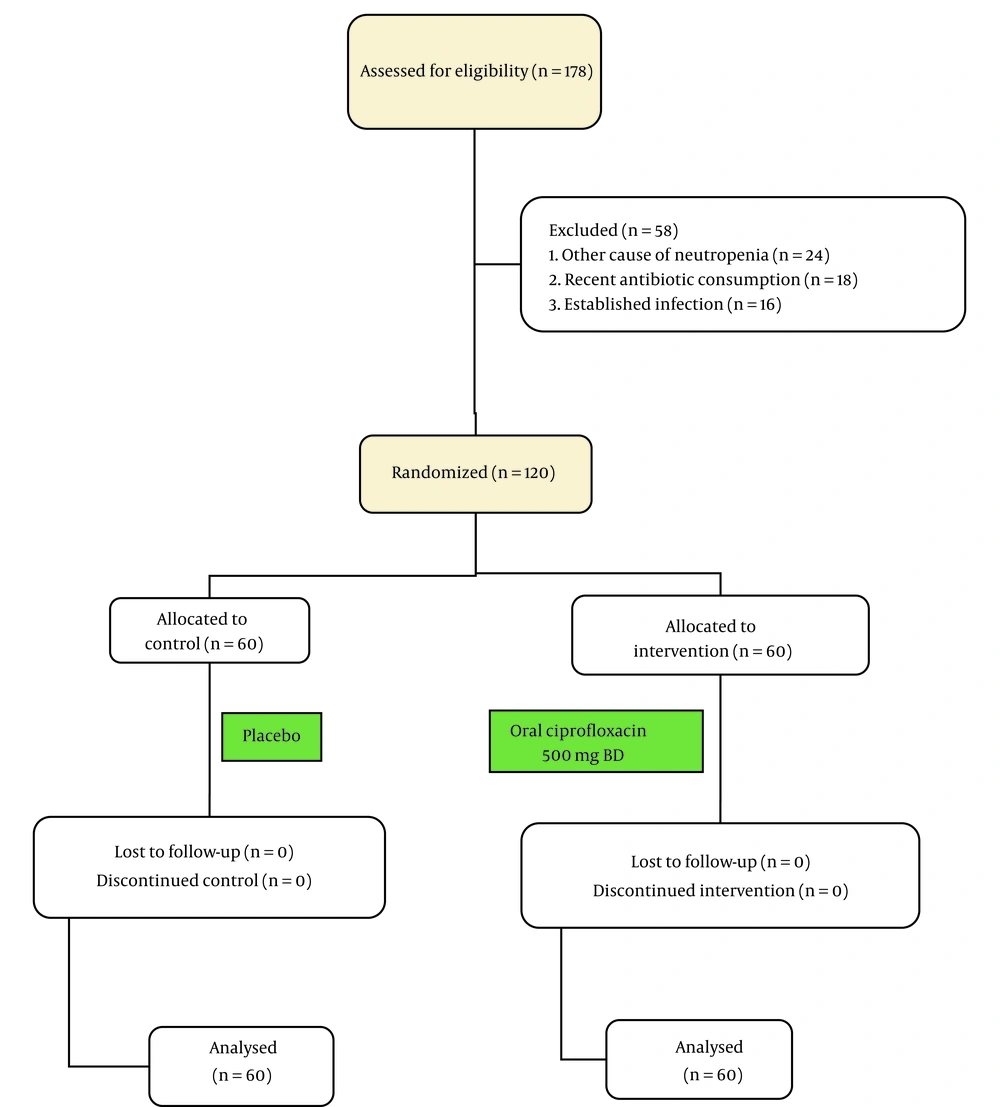
This perspective, double-blind, randomized clinical trial was carried out between 1 March to 1 September 2016 at the hematology-oncology ward of Imam Reza general hospital of AJA Medical University in Tehran, Iran. The eligible participants were non-febrile chemotherapy-induced neutropenic patients. Neutropenia is defined as the neutrophil count below 500 cells per microliter or expected to decrease below 500 in 48 hours. Patients with signs of clinical or microbiological infection, the consumption of antibiotics shortly before neutropenia, and other causes of neutropenia were excluded. This study was approved under the ethical approval code of IR.AJAUMS.REC1392.06. We also registered this study in the Iranian Registry of Clinical Trials [ID: IRCT2015092924266N1, https://irct.ir/trial/20524].
Informed consent was obtained from all participants before they entered the trial executive phase, and they could withdraw from the study at any time. Concerning the 86.2% risk of FN (12) and estimating a 25% decline with antimicrobial prophylaxis, the sample size was calculated at 60 patients in each group. We divided the participants in each arm of the study through the random permuted blocks method. The first arm received oral ciprofloxacin 500 mg twice daily at the occurrence of neutropenia. In contrast, the second arm received similarly shaped manufactured placebo tablets in the same manner by a blinded nurse. We continued ciprofloxacin until the neutrophil count reached 1000 per microliter or fever occurrence, defined as the primary outcome (Figure 1). Another author gathered other data such as age, gender, underlying diseases, type of malignancy, duration of prophylaxis, time of fever, and final prognosis through a questionnaire and direct daily examination. We considered fever (defined as core body temperature ≥38.3°C (101°F) for once or ≥ 38°C (100.4°F) sustained over a 1-h period by an oral mercury thermometer as our primary outcome. We also defined the mortality rate as our secondary outcome. The first authors performed all the clinical examinations, diagnoses, and management to prevent measurement biases. If fever occurred, the patients underwent standard management with a broad-spectrum antibacterial treatment against Pseudomonas spp. infection after bacteriologic studies. We analyzed the findings by SPSS-22 software, IBM Corporation, using chi-square and Fischer's exact test for comparing the demographic characteristics, frequency of fever, mortality rate, and the type of malignancies, and student t-test for comparing the mean age, WBC, chemotherapy cycle and duration between the two groups. All differences were assumed to be significant at P-value < 0.05.
4. Results
There were 73 males (60.8%) and 47 females (39.2%) in our study. The mean age of the patients was 47 ± 14.6 years, with a range between 20 - 83 years. The most common malignancies were acute myeloid leukemia (AML) with 58 of 120 (48.3%) and acute lymphoid leukemia (ALL) with 23 of 120 (19.2%). There were no differences in the demographic characteristics, comorbidities, type of malignancies, primary WBC, chemotherapy duration, and chemotherapy cycle between the two groups by Chi-square and student t-test (Table 1). We compared the frequency of the two groups' primary outcome (fever) and secondary outcome (mortality) by chi-square test. Fever (P = 0.005) was significantly lower in the ciprofloxacin group, but the mortality rate (P = 0.783) was not different between the two groups (Table 2). We also compared the fever frequency between the patients with AML and ALL who received ciprofloxacin, but there was no difference by Fischer's exact test (P = 1.000, Table 3).
| Variables and Intervention | Ciprofloxacin (n = 60) | Placebo (n = 60) | P Value |
|---|---|---|---|
| Gender | 0.262 | ||
| Male | 40 (66.7) | 33 (55) | |
| Female | 20 (33.3) | 27 (45) | |
| Comorbidity | |||
| DM | 10 (16.7) | 13 (21.7) | 0.487 |
| HTN | 12 (20) | 14 (23.3) | 0.658 |
| IHD | 6 (10) | 10 (16.7) | 0.283 |
| COPD | 10 (16.7) | 15 (25) | 0.261 |
| Type of malignancy | 0.107 | ||
| AML | 25 (41.7) | 33 (55) | |
| ALL | 16 (26.7) | 7 (11.7) | |
| Others | 19 (31.6) | 20 (33.3) | |
| Primary WBC | 703 ± 272 | 768 ± 245 | 0.170 |
| Mean age | 45.1 ± 14.9 | 48.8 ± 14.6 | 0.175 |
| Mean days of chemotherapy | 5.2 ± 2.1 | 5.2 ± 1.8 | 0.963 |
| Mean chemotherapy cycle | 3.7 ± 1.9 | 3.8 ± 2.1 | 0.928 |
a Values are expressed as No. (%) or mean ± SD.
| Outcome and Intervention | Ciprofloxacin (n = 60) | Placebo (n = 60) | P Value |
|---|---|---|---|
| Fever | 0.005 b | ||
| Yes | 29 (48.3) | 44 (73.3) | |
| No | 31(51.7) | 16 (26.7) | |
| Mortality | 0.783 | ||
| Dead | 8 (13.3) | 7 (11.7) | |
| Alive | 52 (86.7) | 53 (88.3) |
a Values are expressed as No. (%).
b Statistically significant.
| Intervention and Acute Leukemia | AML (n = 42) | ALL (n = 9) | P Value |
|---|---|---|---|
| Ciprofloxacin | 19 | 4 | 1.000 |
| Placebo | 23 | 5 |
5. Discussion
We studied in this trial the efficacy of ciprofloxacin in preventing FN in patients with cancer and neutropenia. We found that prophylaxis with ciprofloxacin decreases FN but does not influence the mortality rate. Neutropenia is a major cause of severe sepsis and septic shock with considerable mortality. Antimicrobial prophylaxis is a common approach for preventing FN and related mortality in neutropenic patients, but emerging resistant bacteria is a significant challenge in FN prophylaxis (13) and may result in severe sepsis and septic shock with resistant microorganisms. In a study on 2286 patients with gram-negative bacterial septicemia, some risk factors such as a urinary catheter, nephrotic disease, hematologic malignancy, and neutropenia increased in severe sepsis and septic shock (14).
According to the last guideline of the infectious diseases society of America (IDSA), routine antimicrobial prophylaxis is not recommended in neutropenic patients since it does not change mortality rates (15). However, many studies have used antimicrobial prophylaxis in neutropenic patients with success, such as trimethoprim-sulfamethoxazole (16), oral penicillin (17) and first-generation cephalosporins (18). However, they have been widely substituted with fluoroquinolones in recent years. A study found that Gram-positive bacteria accounted for more than half of severe FN episodes in neutropenic patients (19), and fluoroquinolones have comprehensive coverage of gram-negative and gram-positive bacteria (20, 21).
For two decades, FQ drugs have been used in the prophylaxis of fever and bacteremia in chemotherapy-induced neutropenic patients and have had promising results. A study on 8,755 pediatrics undergoing HSCT in Germany showed that prophylaxis with FQ drugs reduced FN episodes and mortality rate (22). Another study of 624 patients with HSCT and acute leukemia in the United States found that prophylaxis with levofloxacin reduced FN in patients with acute leukemia but not in HSCT (23). One study on 1,565 patients with solid tumors and lymphoma in the United Kingdom found that prophylaxis with levofloxacin reduced FN and hospitalization (24). A similar study on 389 Russian patients with various cancers such as leukemia, lymphoma, multiple myeloma (MM), and solid tumors showed similar results with levofloxacin in decreasing FN and mortality (5). In our study, prophylaxis with ciprofloxacin reduced FN episodes in neutropenic patients following chemotherapy. However, it did not change the mortality rates between the two groups. This finding was in agreement with several studies that showed that prophylaxis with FQ drugs reduced FN but did not affect the mortality rate (7, 19, 20, 25, 26). In contrast, some studies showed that prophylaxis with FQ drugs in neutropenic patients did not affect FN or mortality rate, including a study on 69 patients with AML in Iran (27), a study on 86 patients with acute leukemia in Sweden (8) and a study on 69 patients with AML in Mexico (28). Interestingly, one study on 180 patients with leukemia, lymphoma, and solid tumors in Germany found that prophylactic moxifloxacin on neutropenic patients may increase the risk of gram-negative bacteremia (29).
In addition to the above studies, several meta-analyses were performed to evaluate the effect of prophylaxis with FQ drugs on the prevention of FN in neutropenia, including the studies in Israel, the United States, Italy, and Thailand, which showed that prophylactic administration of these drugs in neutropenic patients reduces FN episodes but has no effect on mortality (10, 11, 30, 31). According to a meta-analysis conducted by the European conference on infections in leukemia, FQ did not lower the mortality rate of neutropenic patients but decreased bloodstream infection (32) instead. Another meta-analysis of 113 clinical trials in Canada found that prophylaxis with FQ drugs reduced FN and mortality in neutropenic patients following chemotherapy (33). Antimicrobial prophylaxis decreased FN episodes in neutropenic patients in most studies (11, 20, 21, 24, 28), but the mortality rate was only reduced in a few of the mentioned studies (11, 28). We found similar results in our study, and FN episodes statistically decreased in our trial group, but the mortality rate did not differ between the two groups. Some conditions may affect the selection of prophylaxis regimens, especially malignancy type, drug costs, availability, and antimicrobial resistance. Many studies categorized neutropenic patients as low-risk and high-risk. Concerning this categorization, the prophylactic regimen may differ in neutropenic patients. We used ciprofloxacin for the antimicrobial prophylaxis regimen because of its availability and lower cost than the new generation of FQ drugs. A global guideline for prophylaxis in neutropenic patients may not be available considering the continuous increase in antimicrobial resistance, the difference in antibacterial susceptibility in various regions, different chemotherapy regimens, and host factors. We believe prophylaxis with an FQ drug is helpful in some neutropenic patients, especially those who underwent a high dose of chemotherapy or had a more aggressive type of malignancy, such as acute leukemia.