1. Context
In recent decades, the presence of organic pollutants in water resources has been considered a serious threat to the environment and human health. Pharmaceutical formulations are considered as the pollutants that mainly enter to the surface water and underground resources through the discharge of urban and industrial wastewater. Considering their high stability, pharmaceuticals presence in the environment not only impairs the conventional wastewater treatment processes in the filtration systems, but also exerts toxic effects on humans and other living organisms; hence, removal of these substances has been mainly considered by researchers. Among the wide range of pharmaceutical contaminants, antibiotics have been widely used in medicine and veterinary because of their antimicrobial effects, and thus are of particular importance. These organic compounds enter the environment not only through the effluents from pharmaceutical industries, but also because of lack of metabolism mechanisms during the treatment period. The most significant impact of pharmaceutical compounds is toxicity for microorganisms in the environment as well as disturbance of the ecology balance and microorganisms resistance to antimicrobial and pharmaceutical compounds. Researches have shown that more than 80 different types of active pharmaceutical ingredients have been identified in the environment at concentrations up to several milligrams in the output of municipal wastewater treatment to surface water, groundwater, and drinking water. Concentration of these pollutants in wastewater pharmaceutical companies is almost 100 mg/L. Therefore, although antibiotics are highly useful substances that have been used in many medical and veterinary requirements, they also have undesirable side effects. The main problem in the case of pharmaceutical compounds is their biological activity, leading to adverse effects on the aquatic ecosystem (1).
Although amounts of residual antibiotics are minor, they may create resistance in the population of bacteria, and thus cause antibiotics to become ineffective in the treatment of various diseases. Antibiotics are chemical compounds that inhibit the growth of microorganisms and they often have a biological root, which may be semi-synthetic or fully synthetic (2). Antibiotics are not completely metabolized in the human body after consumption. Their mot metabolized part enters into the wastewater treatment plants through fecal matter and is discharged as the active compounds into the environment. In a study conducted in this field, it was found that 70% of used antibiotics are excreted unchanged; and in most cases, wastewater treatment plants are not capable of refining imported antibiotics and materials enter into the receiving water resources, causing water contamination. Antibiotics are also widely used in animal husbandry, as fertilizers for the agriculture, and they are obtained from animal waste; however, these active pharmaceutical compounds are washed from the surface soil by rainfall and enter into the receiving water. In samples taken from the wastewater, effluent and surface waters downstream of wastewater treatment plants located in urban areas, high concentrations (micrograms/l) of medicinal compounds have been reported (3).
Metronidazole (MTN) is one of the most widely used antibiotics in the world, which has anti-inflammatory and anti-bacterial properties. This antibiotic is classified as nitroimidazole and is used for the treatment of infectious diseases caused by anaerobic bacteria and protozoa, the use of food as an anti-parasite eggs, and fish. Low degradability and high solubility in water are the 2 features of this antibiotic, which make its removal from water by conventional methods difficult and sometimes impossible. Due to the properties of the MTN antibiotic and its adverse effects, the necessity for controlling and removal of this contaminant is highly important (4). In this study, in addition to providing the most widely used methods for eliminating MTN from the contaminated waste, the advantages and disadvantages of each method have also been analyzed.
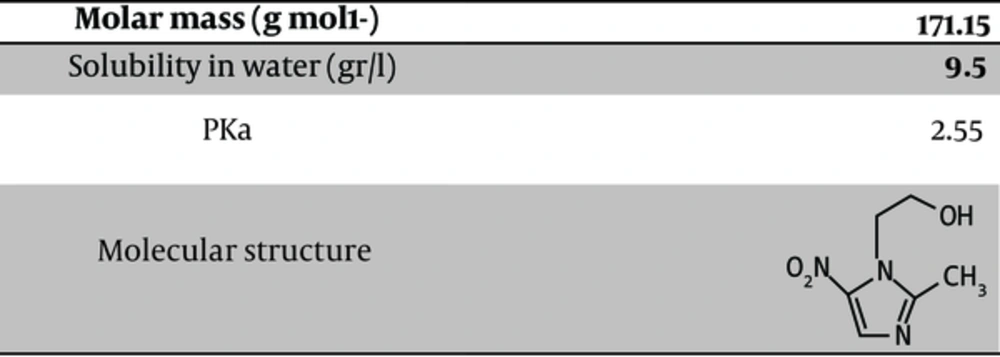
MTN with the chemical formula of C6H9N3O3 is one of the most widely used antibiotics in the world with anti-bacterial and anti-inflammatory properties from the category of nitroimidazole, and it is used to treat infections caused by anaerobic bacteria and protozoa. This chemical is the only drug from nitroimidazole group, which has been placed in the list of essential drugs by the world health organization (WHO) (5). Figure 1 demonstrates the physico-chemical properties of MTN.
Physico-chemical Properties of MTN (1)
The adverse impact of this chemical on the environment and human health has drawn wide attention to the necessity to remove this substance from water (1). Therefore, MTN is one of the mostly used antibiotics in the world. This antibiotic has a ring structure and is potentially carcinogenic and mutagenic for humans due to damage to the DNA of lymphocytes. The international agency for research in a study on the genotoxicity of MTN has reported its carcinogenicity in humans and animals although carcinogenicity of MTN in humans has not yet been confirmed (4). This antibiotic could not be removed using the common methods due to its low degradability and high solubility in water as well as its accumulation in the environment. Therefore it is essential to control and eliminate this contaminant before entering into water resources. Several methods have been suggested for removal of antibiotics from aqueous environments; however, each of the methods has disadvantages along with its advantages, which limit its use (6).
2. Evidence Acquisition
In this descriptive-analytical study, data were collected by reviewing different library resources.
3. Results
3.1. Conventional Methods of Eliminating Metronidazole (MTN) Antibiotics
Various processes can be used to remove antibiotics from water resources.
The use of Gamma ray, adsorption with activated carbon, optical and biological dispersion, membrane system, coagulation, chemical oxidation by ultrasound (US), Fenton (US-O3), US-O3 in the presence of a catalyst different, and O3 in the presence of various nanoparticles are among the common methods, with their own advantages and disadvantages, which are used to remove antibiotics (7). Biological treatment is not effective to remove antibiotics as they eliminate the effective bacteria in the treatment (8). Each of the mentioned methods has a number of disadvantages along with its advantages, which, in most cases, make its use difficult. For example, in the adsorption method, contaminants are collected and separated from the environment, but are transferred only from the liquid phase to the solid phase and are concentrated with no degradation. Moreover, in the physical methods such as coagulation and centrifugation, secondary pollutants are usually produced; biological methods also need much processing time and have low processing efficiency, which are problematic. In contrast, features such as simplicity, low cost, and high efficiency make oxidation processes among common technologies to remove most of the pollutants. So far, several types of oxidation processes for use in water and wastewater treatment have been developed and studied, most of which are based on the use of ozone as the primary oxidant. One of the most important characteristics of ozone is its high oxidation potential (2.07 volts), which has the highest oxidation and electronegativity after fluorine radicals, hydroxyl radicals, and atomic oxygen. Ozone must be in-situ produced; various methods such as photochemical, electrolytes, radiochemistry, and corona discharge can be used for this purpose (9).
Here, the most important and effective methods used to eliminate the antibiotics are briefly described:
3.2. Various Oxidation Methods
Among other processes, advanced oxidation processes are more functional for wastewater treatment from pharmaceutical compounds. The reason is that these methods do not only transfer contamination from one phase to another, but unlike other methods they completely remove pharmaceutical contaminants. In recent years, advanced treatment technologies have been investigated for the removal of pharmaceutical compounds including chemical oxidation with ozone O3/H2O3 membrane filtration and activated carbon adsorption (3). Although these technologies are available, they are not without disadvantages. For example, the oxidation process based on ozone causes decomposition of bromide to bromate ion suspected carcinogen and needs exhaust gas purification and flotation of volatile organic carbon. This is while these problems did not exist through replacing the UV rays rather than ozone in the UV/H2O2 to remove organic pollutants (10).
Therefore, advanced oxidation processes are considered as new methods of removing contaminants from the aquatic environment, which have been used during the past few decades by many researchers as a promising alternative technology for refining wastewater containing organic compounds, especially resistant organic compounds. Nowadays, the use of oxidizing agents such as hydrogen peroxide, persulfate, and periodate have been considered by researchers to increase the functionality of oxidation processes to remove most organic contaminants. Persulfate anion with the chemical formula S2O8-2 has been used as an oxidizing agent with the oxidation – reduction potential to 2.01v – in the process of removal of resistant chemical compounds.
Persulfate as a nonselective anion is a sustainable solution and has specific and unique features such as high speed kinetics, stability at ambient temperature, and less dependence on the type of organic matter. Persulfate is an oxidant that converts a wide range of environmental pollutants in many environmental conditions. Studies have shown the stability of this compound in the environment and its lack of efficacy alone for the removal of organic compounds. It has also been shown that persulfate requires an increase in its activation with the aim of increasing its function in the removal of organic compounds.
UV radiation, US, heat, and divalent metals are among the most important factors in activating persulfate, which ultimately converts persulfate into free radicals of sulfate (SO4) and hydroxyl (OH), with the oxidation reduction potential of 56.2 and 71.2 volt, which is capable of attacking and oxidizing organic compounds based on the 1 to 4 equations (11).
S2O8-2 + ))) → 2SO40-
S2O8-2 + Heat/Light → 2SO40-
2SO40- + H2O → H+ +2SO40- + OH0
Fe2+ + S2O8-2 → Fe3+ + SO40- + SO42-
Nowadays, different methods of oxidation have been excessively studied for the degradation and removal of pharmaceutical compounds including MTN (7). Some studies have revealed the high reactivity of these compounds to ozone. Accordingly, Khataee et al. examined the efficiency of the removal of the MTN antibiotic with oxidation by ozonation and found that ozonation can be an effective method for the removal of MTN and nitroimidazole from the industrial wastewater (9).
3.2.1. Fenton Method
Fenton process is one of the most interesting studied methods among the advanced oxidation processes. Fenton process is divided into different types including dark Fenton, Fenton like process, and photo Fenton. The only difference between Fenton and Fenton like processes is the use of zero or trivalent iron instead of divalent iron in the Fenton process. Fenton process is an electrochemical system, in which Fe2+ ions are as reducing and H2O2 as oxidizing molecules (12). Fenton process caused the process to be easily refined in micro pollutants refinement because of its advantages, which are as follow: the use of simple, short reaction time; high oxidation strength; energy consumption reduction due to catalytic properties; simplicity of the original pollution control of the mineral sector; creation of less toxic waste; the ease of final biological treatment; and the ease of adjusting the working conditions (13). On the other hand, the process is a combination of oxidation and coagulation processes, which leads to production of less sludge, as compared to the processes of coagulation and flocculation. One of the most important oxidation and reduction reactions is ferrous ion reaction with hydrogen peroxide (Fenton) that leads to the generation of hydroxyl radicals. Fenton process is an electrochemical system as reducing molecules Fe+2, in which iron ions are oxidizing (14).
In the Fenton reaction, the main influencing factor is H2O2 that causes organic compounds decomposition (Hydroxyl radicals are produced from reduction of hydrogen peroxide molecules,) (Reactions 1 and 2).
Fe2+ + H2O2 → Fe3+ + OH + OHo
OHo + Organic matter → Oxidation product of organic matter + H2O
3.2.2. Electro-Fenton Process
In recent years, various studies have been conducted on the analysis of xenobiotic such as amoxicillin by advanced oxidation methods such as Fenton, electro, H2O2-UV, O3-H2O, photo Fenton, and electro. These methods are introduced as new technologies and as an alternative or supplement to conventional methods. Although the electro-Fenton has limitations such as corrosion of metals used as the anode, power consumption at higher scale, and color precipitate in solution, it has been more widely used because of its lower overall costs and high efficiency of removal as well as low toxicity of its reagents. One of the other advantages of the electro-Fenton process is removal of organic compounds such as AMX and simple products obtained from degradation by absorbing the hydroxide precipitate in the process. In this process, hydroxyl radicals are produced. In this regard, the research of Cheng et al. (2013) can be noted, in which the performance of the electro-Fenton method in the removal of widely used antibiotic amoxicillin has been assessed. The results revealed that the electro-Fenton method has reasonable efficiency in removing antibiotics from handmade wastewater using iron electrodes in laboratory scale. However, industrial effluent has much lower concentrations than that in the above-mentioned study (14).
3.2.3. The Effect of Ultrasound (US) and UV on Antibiotics Removal
Audio-frequency ultrasonic waves are every wave with frequency above the human hearing range (20 to 40 kHz), which has been used as an antimicrobial agent and for the removal of contaminants as it has high efficiency and does not emit pollution into the environment (6). The main mechanism of this process in the oxidation of pollutants is creation of cavities and microbubbles in water due to the cavitation process, which leads to development of holes in the water and results in the production of about 1000 atmospheric pressure and a temperature of 5000 K, ultimately leading to the formation of hydroxyl radicals. Despite many advantages of US waves, results of several studies in recent years have shown that the application of US waves separately has no usability in a large scale because of their low efficiency, the need for a long time, and limited energy (15). Thus, US waves are used because of their ease of use and lack of production of toxic substances in combination with other methods to increase efficiency and overcome the disadvantages. While using US waves, multiple radicals such as SO4-2, O-2 and H are produced during the activation of per sulfate due to the thermal decomposition of this compound, which has high reactivity feature (16). However, considering the results obtained by Wang et al. during 120 minutes and using per sulfate activating process with US, only 43.88% of carbamazepine were removed. However, the use of stimulating agents such as ozone and divalent metals in the activation process of per sulfate has been considered by researchers with the aim of accelerating the oxidation process. Ozone with redox potential of 2.07 V is one of the oxidants involved in the removal of organic compounds in the environment, which is mainly used in combination with hydrogen peroxide, US, and per sulfate to remove organic materials due to lack of its efficacy if used alone. According to the study of Wang et al. by the combined use of US and ozone, the ozone decomposition occurs in cavitation bubbles, which ultimately leads to the production of powerful hydroxyl radicals (17).
Photocatalytic processes are widely used to remove contaminants (antibiotics) as simple and low-cost alternatives for current approaches such as ozonation and chlorination. In addition, lack of use of toxic materials in this method is one of the advantages. The basis of photocatalytic reactions is that if the semi-conductive particles are irradiated with high-energy radiation, high-energy electron pairs and holes are formed; these generated electron pairs - holes are the initiator of oxidation - reduction process through the production of free radicals, and finally they lead to the mineralization of organic compounds emissions. One of the most important semiconductors is TiO2, which is an affordable, available, non-toxic, and very high performance semiconductor (18).
Reyes et al. (2006) studied the removal of tetracycline antibiotic using TiO2 nanoparticles under UV irradiation and concluded that in general the use of photocatalytic refinement can be used as an interesting topic in degradation of tetracycline antibiotic and replacement of existing conventional. The destruction of tetracycline antibiotic in aqueous solutions by photocatalytic TiO2 irradiated by UV is effective, and degradation mechanism occurs in partnership with holes and hydroxyl radicals (19).
3.2.4. Different Processes using Nanoparticles
Nano-sized compounds have significant physical, chemical, and biological properties that are susceptible to chemical activity and connect with other materials. Among the methods used for loading nanoparticles, hydrothermal and sol-gel methods can be mentioned. Nanoparticles of titanium dioxide alone have poor absorption, and the use of solid adsorbents such as silica, alumina, zeolite, and Murilonits as the support for implantation of titanium dioxide nanoparticles, can increase the absorption of pollutants on the surface of TiO2 nanoparticles, and thus increase absorption efficiency. The properties of TiO2 nanoparticles include low cost, non-toxicity, high oxidation ability, compatibility with the environment, and high surface to volume ratio. Currently, oxidants such as HNO3, KMnO4 and O3 are used to create modified surfaces (14). Nanoparticles have a variety of applications because of their high efficiency in a variety of environmental contaminants including organic compounds, inorganic compounds, pharmaceutical compounds, chlorine compounds, aliphatic compounds, and nitro group (17). Nanoparticles have mechanical, magnetic, optical, electronic, and chemical characteristics due to their very small size and unique molecular or atomic structure. Metals with a capacity of zero include iron, tin, aluminum, and zinc are correction factors for infected aquatic environment.
Among these metals, the use of zero-valent iron (NZVI) has a because of the abundance of inexpensive and non-toxic, rapid response, and high ability, and throughput for analysis of pollutants. In addition, iron particles from industrial processes can be used as zero valent iron in the treatment of pollutants. Researches have shown that iron nanoparticles can be used to remove pollutants such as chlorine, heavy metals, organic, aromatic nitro compounds, poly bromine diphenyl ethers, pesticides, nitrates, and colors (20). Carbon nanotubes are hollow cylindrical structures of graphite flakes, which have high specific surface area, small size, unique crystalline forms, network order, high reactivity, and good mechanical stability. The major problems of using carbon nanotubes and nano-sized adsorbents are their separation after the adsorption process as well as rehabilitation and secondary pollution due to their small size. Therefore, creation of conditions for ease of separation of adsorbents from aqueous solutions is essential (21). Kirmani et al. (2013) examined the removal of MTN using ozonation in the presence of magnesium oxide nanoparticles. The results revealed that the catalytic ozonation process using magnesium oxide nanoparticles can be used as pretreatment or complete treatment plants for waste water or aqueous solutions containing MTN. In addition, magnesium oxide nanoparticles under optimal conditions can be compared to the case where the nan-particle removal efficiency of MTN is added up to 47% (22).
3.2.5. Adsorption Methods and Use of Activate Carbon
Because of their high specific surface area and adsorption capacity, the activated carbon adsorbent surface pores are mainly used to remove organic contaminants from polluted water and sewage. Absorption by activated carbon depends on sorbent properties as well as material properties and environmental pollution of sewage; therefore, factors affecting the absorption of any kind should be assessed for absorption of any pollutants. Granular activated carbon sorbent is suitable for the absorption of antibiotics, especially amoxicillin and has a high absorption capacity. The maximum adsorption capacity with increasing retention time increases absorption. This increase in adsorption could be due to an increase in the number of collisions between the pollutants and adsorbent, and the speed is also a relatively high absorbent (23).
The process of absorption has been more considered in initial cost, reuse of wastewater, simplicity and flexibility in design, easy operation, and non-sensitivity to pollutants and toxic substances as compared to other filtration techniques. The production of wastewater with high quality and formation of free radicals and hazardous materials are considered as other benefits of this method. Among the adsorbents used in the process, activated carbon is more common due to the high volume of pores of adsorption capacity. Many researchers have used activated carbon to absorb antibiotics in aqueous environments. However, the main problem with the use of activated carbon powder, or nano-sized adsorbents, or isolated nanoparticles from solution is the small particle size; hence, the distribution and production of this system is the secondary pollution problem. The magnetism of the adsorbent can be a good solution to solve most of these problems (24).
To eliminate antibiotics with activated carbon adsorption, Mousavi et al. conducted a study and found that absorption potential of granular activated carbon to remove the antibiotic amoxicillin from contaminated water (25).
3.3. Comparison of Some Metronidazole Elimination Methods
In Table 1, the results obtained from some metronidazole elimination methods are presented.
| Antibiotic | Concentration | Matrix | Treatment | Operating conditions | Results and comments | References |
|---|---|---|---|---|---|---|
| Metronidazole | 10 - 30 mg/L | Distilled water, natural waters and wastewater | Simultaneous | pH = 2- 9 | The ozonation degradations were higher than 90% and 10- 20% of TOC removal. - Ozonation generates highly toxic oxidation by-products. | (26) |
| Application of ozonation and adsorption | 0.25- 0.50 g/L activated carbon | - The presence of activated carbon during the ozonation produces an increase in the removal rate, a reduction in the toxicity of oxidation by-products and a reduction of around 30% in the TOC. | ||||
| Metronidazole | 100 - 600 mg/L | Distilled water, Natural waters and wastewater | Adsorption/Bioadsorption on activated carbon | T = 25 C; pH = 2- 11 | The pH of the medium and the electrolyte concentration did not influence the adsorption removal. | (27) |
| 0 - 0.1 M NaCl | - Antibiotics were not degraded by the microorganisms used in the biological treatment. | |||||
| 1 g/L activated carbon | - The presence of these microorganisms during the adsorption increases their adsorption/ bioadsorption on the activated carbon. | |||||
| Metronidazole | 150 mg/L | Distilled water | Adsorption on activated carbons | T = 25 C; 0.2 - 1 g/L activated carbon | 90% removal was achieved with 1 g/L of activated carbon. | (28) |
| pH = 7 | - 2nd order kinetic fits suitably the experimental data | |||||
| Metronidazole | 1 mg/L | Deionised water | Direct and indirect Photolysis | Photolysis: LP UV at 254 nm | Photo-degradation exhibited pseudo 1st order kinetics. | (12) |
| Fenton | MP UV at 200 - 400 nm; pH = 6; 0 - 50 mg/L; H2O2 | - MP irradiation was more effective than LP. | ||||
| Photo-Fenton | Photo-and Fenton: LP UV at 254 nm; pH = 3.5; 29.4 mM H2O2; 2.94 - 11.76; mM Fe2+ | - Direct photolysis (6% - 12% removal) was less effective than UV/H2O2 oxidation (58% - 67% removal). | ||||
| - Fenton oxidation followed 2nd order kinetics and the rate was increased with High Fe2+ concentrations. | ||||||
| - An increase in the removal efficiency and in the reaction rate occurred when photo-Fenton (74% - 94% removal) is compared to Fenton oxidation (53% - 76% removal). | ||||||
| Metronidazole | 1 - 25 mg/L | Deionised water | adsorption on various carbon materials | pH = 2 - 12; temperature, = 15-35 C°; NaCl = 0.1 - 1 N | - The adsorption of MNZ was enhanced when the MNZ solutions were prepared using wastewater. Therefore, the electrolytes present in the wastewater cooperated rather than competed with the MNZ molecules for the adsorption sites. | (1) |
| - Desorption equilibrium data of MNZ on all carbon materials demonstrated that the adsorption was reversible corroborating the weakness of the adsorbent-adsorbate interactions. | ||||||
| Metronidazole | 40 - 120 mg/L | Deionised water | Photocatalytic degradation | dosage (0.5 - 3 g/L) pH = 3 - 11 | - Maximum removal of MNZ was observed at near neutral pH. | (29) |
| A 125 W medium-pressure UVC lamp emitting maximum wavelength at 247.3 nm and a low-pressure UV lamp with irradiation intensity 8 W were applied as light sources | - Removal efficiency was decreased by increasing dosage and initial MNZ concentration. | |||||
| - The reaction rate constant (kobs) was decreased from 0.0513 to 0.0072 min-1 and the value of electrical energy per order (EEo) was increased from 93.57 to 666.67 (kWh/m3) with increasing initial MNZ concentration from 40 to 120 mg/L, respectively. | ||||||
| - The biodegradability estimated from the BOD5/COD ratio was increased from 0 to 0.098. |
4. Discussion
Pharmaceutical compounds can cause significant risks to amphibious organisms entering the environment. Although there are minor amounts of residual antibiotics, they may create resistance in bacteria populations, and thus cause antibiotics to become ineffective in treating different diseases (26). Antibiotics and pharmaceutical compounds have been widely used to treat infectious bacterial diseases that are often not removed using conventional wastewater treatment processes. Presence of antibiotics and pharmaceutical compounds in the environment as emerging contaminants has created considerable concern (6).
Different groups of antibiotics are found in groundwater, surface water, sewer, water, and even soil. Pharmaceuticals or their metabolites enter into the municipal sewage through their use and disposal by humans. Because the operation of the wastewater treatment refinery is not enough to remove such materials, they enter into receiving waters without adequate treatment, causing environmental pollution and threatening public health; such materials may even enter rivers and lakes and endanger aquatic life. Because serious monitoring is not performed to control antibiotics in refineries, the routine conventional processes such as BOD, COD are not responding. In general, it appears that these processes should be integrated to have more effectiveness. However, each of these methods has its own advantages and disadvantages; however, in recent years, the advanced oxidation process has been effective, as it relies on the production of free radicals and active radicals, especially OH0, and has high oxidation power. Several types of oxidation processes have been developed for use in water and wastewater treatment, and most of them have been reported to be based on the use of ozone as the primary oxidant. As its most important characteristic, ozone has the highest oxidation and electronegativity after fluorine radicals, hydroxyl radicals, and atomic oxygen.
4.1. Conclusions
According to the results of this investigation, it appears that in recent years, nanotechnology and the advanced oxidation process, which are based on the production of free and active radicals, especially OH0, have been effective because of their high oxidation power.