1. Background
Obesity is usually associated with mild inflammation and metabolic syndrome.
Many people are overweight and obese, and obesity is becoming an epidemic in developed societies (1). Inactivity and excessive consumption of high-calorie food are the most important causes of obesity. These factors can cause cardiovascular diseases, type 2 diabetes, and some types of cancer and reduce the quality of life (2). Other factors involved in cardiovascular diseases include inflammation, oxidative stress, endothelial cell activity, and platelet activity. Symptoms of cardiovascular diseases include inflammatory indicators (such as fibrinogen, adhesion molecules, amyloid A, serum interleukin, and acute phase proteins) (3). Metabolic syndrome includes a combination of hypertension, glucose metabolism disorder, fat metabolism disorder (dyslipidemia), and obesity (especially abdominal obesity) (4). Increased consumption of carbohydrates, especially refined sugars that have high fructose causes metabolic syndrome (5). To prevent and treat metabolic syndrome and related inflammations, regular physical activity is recommended (6). Various studies have shown that aerobic exercises reduce fat mass and inflammation in the body (7). The Pentraxin family includes two sets of long pentraxin and short pentraxin. Examples of short pentraxin include C-reactive protein (CRP) and serum amyloid, which are produced in the liver. A complete example of the long pentraxin family is pentraxin-3 (PTX-3) (8). Pentraxin 3 is expressed by various types of tissues and cells and is especially produced by innate immune cells in response to pro-inflammatory signals (9). PTX3 plays a role in maintaining vascular tone and cardiovascular function (10, 11). PTX3 is associated with vascular inflammation (12). However, both pro-inflammatory and anti-inflammatory properties have been reported in clinical models. Anti-inflammatory cytokines and atheroprotective signals, for example, interleukin-10 (IL-10) and high-density lipoprotein (HDL), are able to induce PTX3 expression (13).
Other inflammatory biomarkers effective in the process of atherosclerosis are adhesion molecules, the main of which is vascular cell adhesion molecule-1 VACM-1. This protein is secreted in response to CRP activities. VACM-1 is one of the most sensitive cell markers in the formation of atherosclerotic plaque in the vascular endothelial wall (14). In studies related to metabolic syndrome, the intensity of physical activity is considered an important factor (15). High-intensity interval training (HIIT) is important both in terms of time-saving and is more effective in losing weight and consuming fat. HIIT training protocols are varied and typically include a short, repetitive phase of high-intensity exercise immediately followed by a period of rest or low-intensity exercise (16). It has been shown that high-intensity interval training can improve cardiovascular fitness in patients with coronary heart disease, people with metabolic syndrome, and obese people (17).
2. Objectives
Metabolic syndrome and related diseases have harmful effects on the health and economy of society. In addition, inactivity and lack of time to exercise have increased obesity and metabolic syndrome. Therefore, research on the role of high-intensity interval training on metabolic syndrome indicators and inflammatory indicators (vascular inflammation and systemic inflammation) is considered necessary and important.
3. Methods
3.1. Animals
In this study, 37 male Wistar rats weighing 220 ± 20 g were used. Rats were kept under the standard conditions of a temperature of 22 ± 2°C and humidity of 45%, and consecutive 12-hour periods of light and darkness. Water and food were checked daily, and they were provided with standard rat-specific water and food. Also, their cages were cleaned regularly twice a week.
3.2. Study Methodology
The Wistar rats under study were divided into five groups by a simple random method after a week of familiarization with the laboratory environment as follows:
(1) Experimental group 1: This group included 7 male Wistar rats that were subjected to a high-calorie fructose diet. This group received a high-calorie diet for 6 weeks from the 11th week of life, and after the end of these 6 weeks and subsequent 12 hours of food deprivation, they became unconscious and then were dissected.
(2) Experimental group 2: This group also included 7 male Wistar rats that were subjected to a high-calorie fructose diet. This group received a high-calorie diet for 6 weeks from the 11th week of life, and after the end of these six weeks, they did high-intensity interval training on a treadmill for eight weeks and had a normal diet. At the end of eight weeks and following 12 hours of food deprivation, they became unconscious and then were dissected.
(3) Experimental group 3: This group also included 7 male Wistar rats that were subjected to a high-calorie fructose diet. This group was given a high-calorie diet for 14 weeks from the 11th week of life, and after the end of these 14 weeks, followed by 12 hours of food deprivation, they became unconscious and then were dissected.
(4) Control group 1: This group also included 7 male Wistar rats that did not participate in any exercise or nutritional intervention. This group received a normal diet from the eleventh week of life for six weeks as a control group, and after the end of the sixth week and subsequent 12 hours of food deprivation, they became unconscious and were then dissected.
(5) Control group 2: This group also included 7 male Wistar rats that did not participate in any exercise or nutritional intervention. This group received a normal diet from the 11th week of life for 14 weeks as a control group, and after the end of the 14th week and the following 12 hours of food deprivation, they became unconscious and were then dissected.
The exercise protocol included 8 weeks of interval training consisting of high and low-intensity stages:
High-intensity stages included 2 minutes with an intensity of 75% of the maximum speed in the first week, 80% of the maximum speed in the second week, 85% of the maximum speed in the third week, 90% of the maximum speed in the fourth week was the end of the training period (Table 1) (18).
| Exercise Protocol | First Week | Second Week | Third Week | Fourth Week | Fifth Week | Sixth Week | Seventh Week | Eighth Week |
|---|---|---|---|---|---|---|---|---|
| Intense training intensity, percentage of maximum speed | 75% | 80% | 85% | 90% | 90% | 90% | 90% | 90% |
| Intensity of slow training, percentage of maximum speed | 30% | 30% | 30% | 20% | 20% | 20% | 20% | 20% |
| The number of practice sessions | 2 | 4 | 6 | 8 | 8 | 8 | 8 | 8 |
High-Intensity Interval Training Protocol (HIIT)
Low-intensity stages included two minutes with an intensity of 30% of the maximum speed from the first week to the end of the third week and 20% of the maximum speed from the beginning of the fourth week to the end of the training period (Table 1) (18).
3.3. High-Calorie Diet
At the end of the first week (familiarization period), a high-calorie diet (normal diet with fructose solution) was given to the three experimental groups and a normal diet to two control groups.
Experimental groups were given 100 grams per liter of fructose with water for six weeks.
The total calorie intake of the control groups was 2.89 kilocalories per kilogram of body weight, but in the experimental groups, it was 4 kilocalories per kilogram of body weight (19).
3.4. Evaluation of Research Variables
In order to eliminate the acute effect of exercise, sampling was done 48 hours after the last exercise session. Rats were anesthetized by a compound intraperitoneal injection of ketamine (70 mg/kg) and Xylosin (3 - 5 mg/kg). Blood samples were taken from the superior vena cava and collected in Falcon tubes. Collected samples were centrifuged at a speed of 4000 rounds per minute for 15 minutes at a temperature of 4 degrees Celsius, and the serum was separated. The concentrations of PTX3, VACM-1, and CRP in serum were measured by ELISA method using special kits for rats (for CRP from Cusabio Biotech, Wuhan Company in Chinese, and for PTX3 and VACM-1 from Diaclone, Besancon Company in France, respectively).
3.5. Statistical Analysis
To analyze the data, the Shapiro-Wilk, independent t-test, one-way analysis of variance (ANOVA), and Bonferroni post-hoc tests were used (P ≤ 0.05).
4. Results
According to the definition of metabolic syndrome, if at least three disorders of obesity disorders, high levels of TG and LDL, low HDL, increased fasting glucose, and increased blood pressure occur, it can be claimed that this syndrome has been established. The results obtained in this regard are presented in Table 2.
| Groups and Variables | Mean + SD | t | Significance |
|---|---|---|---|
| FG-6 | |||
| Weight, g | 13.80 ± 235.12 | 3.84 | 0.068 |
| HDL, mmol/L | 39.63 ± 3.40 | 4.15 | 0.041 a |
| LDL, mmol/L | 24.70 ± 3.61 | 5.53 | 0.012 a |
| TG, mmol/L | 84.46 ± 14.69 | 5.27 | 0.019 a |
| Fasting glucose, mg/dL | 121.52 ± 4.19 | 2.45 | 0.105 |
| FG-14 | |||
| Weight, g | 269.18 ± 16.09 | 5.18 | 0.015 a |
| HDL, mmol/L | 27.31 ± 5.29 | 8.64 | 0.001 a |
| LDL, mmol/L | 56.30 ± 4.33 | 9.23 | 0.001 a |
| TG, mmol/L | 72.19 ± 11.29 | 7.78 | 0.001 a |
| Fasting glucose, mg/dL | 161.49 ± 7.35 | 7.24 | 0.007 a |
Percentage Changes of Variables at the End of the Sixth and Fourteenth Week
A significant increase in TG and LDL levels and a significant decrease in HDL levels at the end of the sixth week, as well as a significant increase in body weight, TG and LDL levels, a significant decrease in HDL levels, and an increase in fasting glucose at the end of the fourteenth week, indicated the development of the syndrome metabolic at the end of the sixth week and with more intensity at the end of the fourteenth week (Table 2).
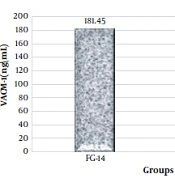
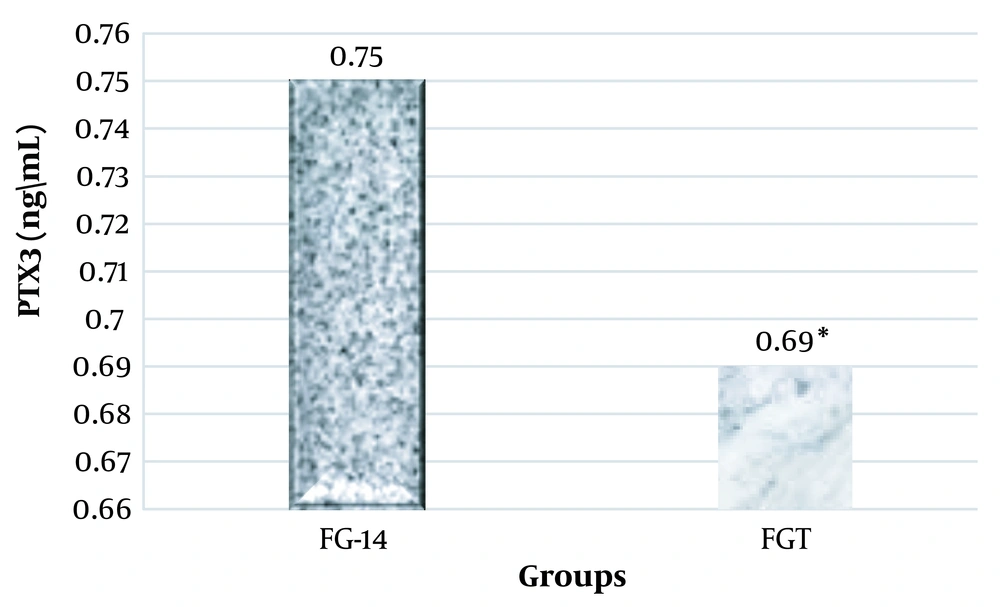
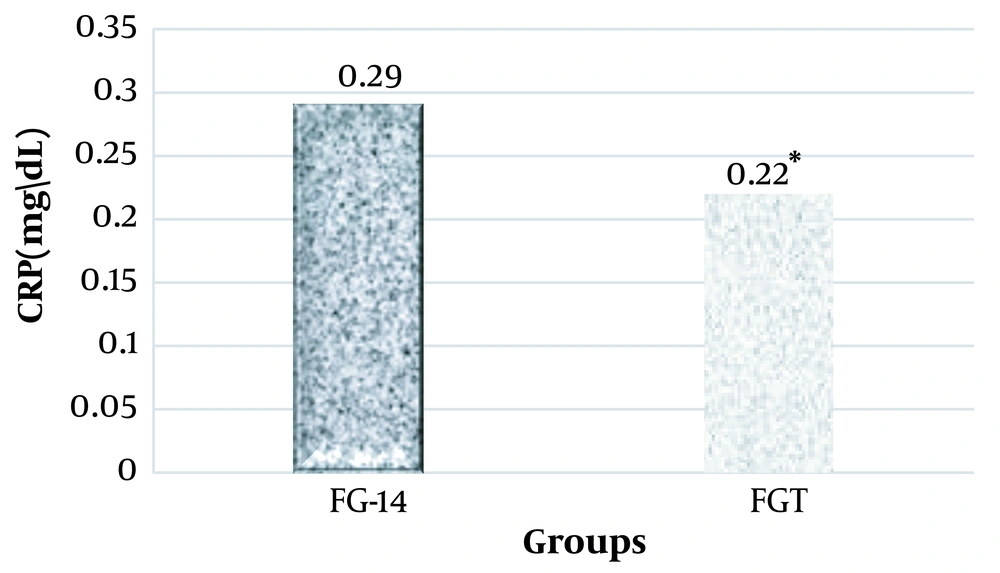
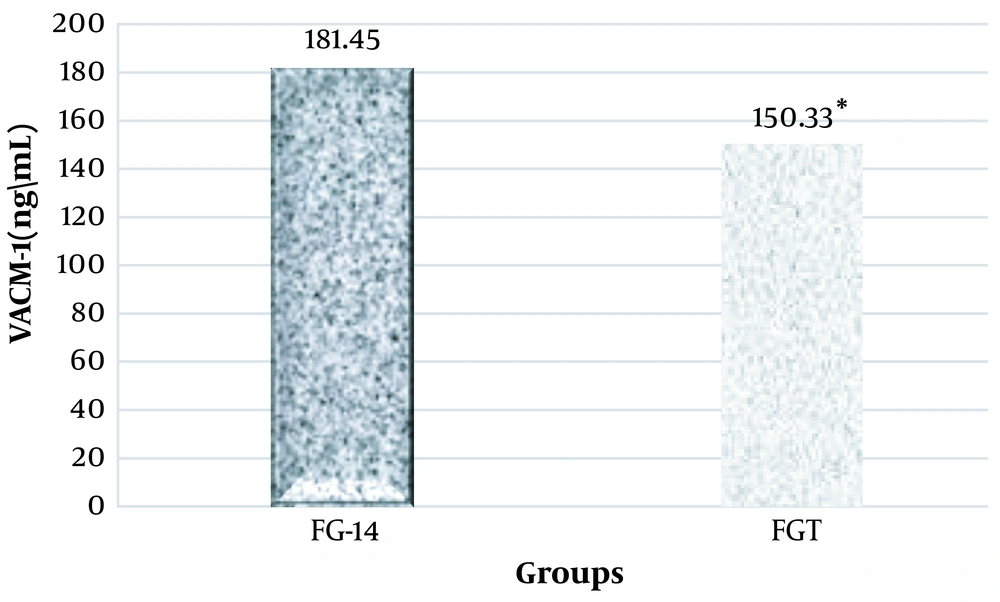
The results of the one-way analysis of variance showed a significant increase in the plasma levels of PTX3, VACM-1, and CRP at the end of the sixth week and the fourteenth week in the groups that were on the fructose diet (Table 3).
Results of Analysis of Variance for Variables PTX3, VACM-1, and CRP in Groups at the End of the Fourteenth Week
Considering that in this research, the effect of high-intensity interval training on PTX3, VACM-1, and CRP plasma levels in rats suffering from metabolic syndrome was investigated, two groups of FG-14 and FGT were compared using the independent t-test method. It was observed that high-intensity interval training had a significant effect on the plasma levels of PTX3 (ng/mL), VACM-1, and CRP in male Wistar rats and significantly decreased their levels (Figures 1, 2, and 3).
5. Discussion
The results of this research were consistent with the results of the Ameral et al. study (20). Ameral et al. used 20 four-week-old rats, and, to induce metabolic syndrome, rats received fructose as a drink in combination with water (100 g/L for 18 weeks). Then the subjects were divided into control groups, metabolic syndrome, metabolic syndrome + exercise (walking), and metabolic syndrome + exercise (running) groups. Both training groups (walking and running) started training from the ninth week (5 days a week, 60 minutes each day for 9 weeks). It was observed that animals that received high-dose fructose had significantly higher levels of glucose, triglycerides, and insulin resistance. It seems that the entry of high fructose into the liver, which is the main organ for metabolizing fructose, caused a change in the metabolic pathways and, as a result, increased lipogenesis in the liver, production, and accumulation of triglycerides in the liver, and insulin resistance. All these have caused metabolic syndrome in the rats of the present study (21).
Insulin resistance caused by high fructose consumption is usually associated with an increase in triglyceride concentration and a decrease in HDL concentration (20). Research results show that a high intake of refined carbohydrates may increase the risk of developing insulin resistance. Long-term consumption of fructose in the diet can increase the activity of liver lipogenic enzymes, and the synthesis of lipids increases the levels of VLDL and triglycerides (22).
Also, the results of our research were consistent with the results of Herawati et al., 2020, who investigated the effect of a diet with a high glycemic index (GI). Herawati et al. divided rats into two control groups with a standard diet and a high blood glucose index (HighGL) group. The results of their research showed that the body weight showed a significant change before and after receiving a diet with a high glycemic index, and blood glucose levels increased significantly (23).
In fact, fructose is converted into fructose 1-phosphate in the liver and causes the separation of glucokinase from its regulatory protein, and, as a result, it leaves the nucleus.
This action may be an opposing factor that sometimes increases with high dietary fructose consumption and excessive use of carbohydrates and fat synthesis in the liver (24).
Also, the results of this research showed that the induction of metabolic syndrome led to a significant increase in the plasma levels of PTX3, VACM-1, and CRP. In fact, inflammation is the connection point between obesity and metabolic syndrome (25). Chronic inflammation is associated with metabolic syndrome and obesity. During inflammation, oxidative stress increases while the metabolic balance is not maintained accordingly, and consequently, the disease progresses (26). Recent studies show that inflammation plays an essential role in various stages of atherosclerosis, such as the beginning and progression and formation of atheroma, instability and rupture of platelets, and vessel stenosis after angioplasty. Adhesion of leukocytes, neutrophils, and monocytes to the endothelium and then the migration of leukocytes through the endothelium into vessel walls is one of the main characteristic stages of the inflammation process. The results of these studies show the relationship between inflammation and cardiovascular diseases (27).
Proteins with alternating concentrations in the plasma and blood serum of humans and animals due to inflammation, necrosis, and bacterial and viral infections are called acute phase proteins or APPs.
The role of these proteins is to reduce the inflammatory effects in the tissues, remove inflammatory agents, remove and destroy damaged tissue parts, and finally restore the tissue. Therefore, it can be concluded that metabolic syndrome in rats leads to inflammation. To fight this inflammation, the body will start to make and secrete acute-phase proteins in the liver using the innate immune system (28). The results of the present study demonstrated eight weeks of high-intensity interval training significantly reduced the plasma levels of PTX3, VACM-1, and CRP in male Wistar rats. One of the mechanisms involved in this is the reduction in the production of cytokines secreted from fat tissue (29). Losing weight and increasing physical activity can affect the immune system by reducing pro-inflammatory cytokines such as TGFB, TNFR, TNF-α, IL-8, and IL-6. Reducing fat tissue will not only reduce the volume of adipocytes and fat precursor cells but also reduces the number of remaining endothelial cells and macrophages. These cells produce pro-inflammatory mediators such as CRP, serum amyloid protein (SAA), and some cytokines. Weight loss and exercise can increase the gene expression of anti-inflammatory mediators such as IL-10 and IL-1 receptor antagonists in cells. As a result, since the liver is involved in fat accumulation and metabolism, it participates in reducing the production of fibrinogen and pro-inflammatory mediators (29).
Regular daily exercise has anti-inflammatory and protective effects against cardiovascular diseases and leads to a decrease in serum CRP (30). The possible mechanism of the decrease in CRP levels after exercise may be due to the decrease in the concentration of interleukins after repeated exercise. One of the cytokines is IL-6, which is secreted by adipose tissue and stimulates liver cells to produce CRP (7). PTX3 is secreted in inflammation and various types of cells, including skeletal muscle, monocytes, macrophages, endothelial cells, and smooth muscle cells, as well as in atherosclerotic wounds (31).
The main stimulus for the production of PTX3 in the human body is TNF-α. TNF-α plasma levels decrease due to long-term exercise; therefore, decreasing the levels of TNF-α will decrease the levels of PTX3. On the other hand, regular and long-term sports activity limits myeloperoxidase produced by neutrophilsm. Myeloperoxidase is secreted from myeloid cells in neutrophils, and its excessive production causes atheroma formation, rupture of atherosclerotic plaques, and acute coronary events. Therefore, the limitation of myeloperoxidase as a result of long-term sports activity reduces the production of PTX3 by neutrophils. In addition, regular exercise reduces the capacity of monocytes to release PTX3 (32).
In relation to reducing the levels of vascular adhesion molecules due to interval training, it should be said that interval training decreased the production of TNF-α by increasing the activity of the sympathetic system and increasing anti-inflammatory cytokines. Due to the fact that the secretion of adhesion molecules is stimulated due to the increase in the secretion of inflammatory cytokines, the reduction of pro-inflammatory factors reduces the release of chemical mediators and the reduction of pro-inflammatory transcription factors, such as nuclear factor kappa (NF-Kβ), which can be effective in modulating vascular inflammation, and, subsequently, the concentration of adhesive molecules will decrease (33). Another mechanism includes the release of catecholamines in the blood circulation after exercise, in a manner that, with the increase of epinephrine, usually the serum levels of adherent cells decrease (34). In addition, the beneficial effects of exercise on endothelial function include the increase of plasma HDL-c levels, which can lead to the release of prostacyclin-2 (PGL-2) from muscle cells and prevent plaque accumulation and reduce vascular adhesion molecules in the body (35).
5.1. Conclusions
Induction of fructose diet-induced metabolic syndrome in male Wistar rats, and this metabolic syndrome significantly increased the plasma levels of PTX3, VACM-1, and CRP. However, eight weeks of interval training significantly reduced the plasma levels of PTX3, VACM-1, and CRP in rats with metabolic syndrome.